Abstract
Objective
To assess the safety and immunogenicity of pneumococcal conjugate vaccine (PCVs) in preterm infants.
Methods
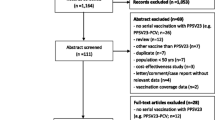
In accordance with the PRISM (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (as of May 2015), a meta-analysis was conducted to evaluate the safety and immunogenicity of PCVs in preterm infants.
Results
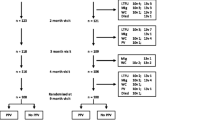
Ten thousand nine hundred sixty full-term infants and 2131 preterm infants with 344 preterm infants of <2500 g birth weight [low-birth weight (LBW)] were included, and all the subjects were immunized with either PCV-7, PCV-10 or PCV-13 in this random-effects meta-analysis. For safety, the range of risk ratio (RRs) for local reaction was from 0.88 to 1.02 and from 0.94 to 1.24 for systematic reaction respectively. For immunogenicity, either post-primary or booster vaccination with PCV-7, PCV-10 or PCV-13, genomic mean concentration (GMC) of serotypes 4, 6B, 9 V, 19F and 23F was always less in preterm infants than in full-term infants, in which huge comparison of GMC was found in serotype 19F(SMD = −0.393, 95%CI:-0.612 ~ 0.175). After primary vaccination, the combined risk ratio (RRs) of immune response against seven common serotypes and additional serotype 1 was approximated to 1.00 with narrow 95 % confidence interval (CI) between preterm infants and full-term infants, and at least 91 % sero-conversion of two additional serotypes, 5 and 7F in two cohorts was observed. Furthermore, between very-low-birth-weight (VLBW) infants of <1500 g and 1501 ~ 2500 g, overall RRs of immune response to PCV-7 administration was 0.98 (95%CI: 0.96 ~ 1.00).
Conclusions
Preterm infants have a great tolerance to PCV-7, PCV-10 or PCV-13 vaccination. PCV-7 could elicit optimal immune response post vaccination in preterm infants, even in VLBW infants.



Similar content being viewed by others
References
Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94.
Baxter D, Ghebrehewet S, Welfare W, Ding DCD. Immunizing premature infants in a special care baby unit in the UK. Results of a prospective, non-inferiority based, pragmatic case series study. Hum Vaccine. 2010;6:512–20.
Slack MH, Schapira D, Thwaites RJ, et al. Acellular pertussis vaccine given by accelerated schedule: response of preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F57–60.
Tsuda K, Iwasaki S, Horiguchi H, et al. Immune response to Haemophilus influenzae type b conjugate vaccine in preterm infants. Pediatr Int. 2012;54:64–7.
Collins CL, Ruggeberg JU, Balfour G, et al. Immunogenicity and immunologic memory of meningococcal C conjugate vaccine in premature infants. Pediatr Infect Dis J. 2005;24:966–8.
Lee LH, Gu X-X, Nahm MH. Towards new broader spectrum pneumococcal vaccines: the future of pneumococcal disease prevention. Vaccines. 2014;2:112–28.
Pneumococcal vaccines WHO position paper – 2012. Wkly Epidemiol Rec. 2012;87:129–44.
Centers for Disease Control (CDC). Update: pneumococcal polysaccharide vaccine usage--United States. MMWR Morb Mortal Wkly Rep. 1984;33:273–6, 281.
Lee H, Choi EH, Lee HJ. Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines. Korean J Pediatr. 2014;57:55–66.
Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente vaccine study center group. Pediatr Infect Dis J. 2000;19:187–95.
Gadzinowski J, Albrecht P, Hasiec B, et al. Phase 3 trial evaluating the immunogenicity, safety, and tolerability of manufacturing scale 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29:2947–55.
Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866–75.
Szynczewska E, Chlebna-Sokół D. Immunogenicity of heptavalent conjugate vaccine against Streptococcus Pneumoniae in premature babies with low birth weight. Pediatr Neonatol. 2014;55:101–7.
D’Angio CT, Heyne RJ, O’Shea TM, et al. Heptavalent pneumococcal conjugate vaccine immunogenicity in very-low-birth-weight, premature infants. Pediatr Infect Dis J. 2010;29:600–6.
Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in lowbirth weight and preterm infants. Pediatr Infect Dis J. 2002;21:182–6.
Rückinger S, van der Linden M, von Kries R. Effect of heptavalent pneumococcal conjugate vaccination on invasive pneumococcal disease in preterm born infants. BMC Infect Dis. 2010;10:12.
Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol. 2010;17:134–42.
Jie L, Wendong L. Theroy & practice of systematic review/meta-analysis. 1st ed. China: Military Medical Science Press; 2013. p. 106–8.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Higgin JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration 2011. Available at: www.cochrane-handbook.org.
Moss SJ, Fenton AC, Toomey JA, Grainger AJ, Smith J, Gennery AR. Responses to a conjugate pneumococcal vaccine in preterm infants immunized at 2, 3, and 4 months of age. Clin Vaccine Immunol. 2010;17:1810–6.
Esposito S, Pugni L, Bosis S, et al. Immunogenicity, safety and tolerability of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months post-natally to pre- and full-term infants. Vaccine. 2005;23:1703–8.
Szynczewska E, Chlebna-Sokół D. Immunogenicity and safety of heptavalent conjugate vaccine against Streptococcus Pneumoniae in pre-term polish infants. Vaccine. 2011;29:7107–13.
Ruggeberg JU, Collins C, Clarke P, et al. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine. 2007;25:264–71.
Omeñaca F, Merino JM, Tejedor JC, et al. Immunization of preterm infants with 10-valent pneumococcal conjugate vaccine. Pediatrics. 2011;128:e290–8.
Martinón-Torres F, Czajka H, Center KJ, et al. 13-valent pneumococcal conjugate vaccine (PCV13) in preterm versus term infants. Pediatrics. 2015;135:e876–86.
Chlebna-Sokó D, Szynczewska E. Evaluation of the immune response to vaccination against pneumococcus in children born prematurely including the influence of perinatal factors. HK J Paediatr. 2015;20:17–24.
Woestenberg PJ, van Lier A, van der Maas NA, Drijfhout IH, Oomen PJ, de Melker HE. Delayed start of diphtheria, tetanus, acellular pertussis and inactivated polio vaccination in preterm and low birth weight infants in the Netherlands. Pediatr Infect Dis J. 2014;33:190–8.
Mutua MK, Ochako R, Ettarh R, Ravn H, Echoka E, Mwaniki P. Effects of low birth weight on time to BCG vaccination in an urban poor settlement in Nairobi, Kenya: an observational cohort study. BMC Pediatr. 2015;15:45.
Calderón CG, Moore VR, Pittaluga PE, Potin SM. Adherence to immunizations in newborns less than 1500 gr at birth and/or younger than 32 weeks, in two chilean center. [Article in Spanish]. Rev Chilena Infectol. 2011;28:166–73.
Ochoa TJ, Zea-Vera A, Bautista R, et al. Vaccine schedule compliance among very low birth weight infants in lima, Peru. Vaccine. 2015;33:354–8.
Ruiz-Aragón J, Márquez Peláez S, Molina-Linde JM, Grande-Tejada AM. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in infants: a meta-analysis. Vaccine. 2013;31:5349–58.
Chen CY, Chen HL, Chou HC, Tsao PN, Hsieh WS, Chang MH. Weight-based policy of hepatitis B vaccination in very low birth weight infants in Taiwan: a retrospective cross-sectional study. PLoS One. 2014;9:e92271.
Stumpf KA, Thompson T, Sánchez PJ. Rotavirus vaccination of very low birth weight infants at discharge from the NICU. Pediatrics. 2013;132:e662–5.
Davis, RL1, Rubanowice D, Shinefield, HR, et al. Immunization levels among premature and low-birth-weight infants and risk factors for delayed up-to-date immunization status. Centers for Disease Control and Prevention Vaccine Safety Datalink Group. JAMA. 1999;282:547–53.
Acknowledgments
The authors thank MD. Carl T. D’Angil for providing original data on GMC against PCV-7 in LBW infants in his study.
Contributions
KD designed this study and processed the original data; JG and PL collected and reviewed the publications. KD will act as guarantor for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
KD and JG are employees of Wuhan Institute of Biological Products Co. Ltd., a company developing and selling human vaccine; but the company had no influence on the design and execution of this present study.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Duan, K., Guo, J. & Lei, P. Safety and Immunogenicity of Pneumococcal Conjugate Vaccine in Preterm Infants: A Meta-Analysis. Indian J Pediatr 84, 101–110 (2017). https://doi.org/10.1007/s12098-016-2248-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-016-2248-2