Abstract
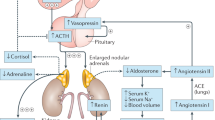
Congenital adrenal hyperplasia (CAH) is a common disorder of impaired adrenal cortisol biosynthesis with associated androgen excess. The clinical presentation of 21-hydroxylase deficiency, the commonest cause of CAH, forms a spectrum and can be divided into classic and non-classic types. The former consists of life threatening salt wasting and non-life threatening simple virilizing phenotypes. Patients with the non-classic form are asymptomatic or have mild features of androgen excess. Most developed countries have newborn screening facilities for CAH. In the absence of newborn screening, the diagnosis of CAH may be missed or delayed. This can result in neonatal mortality in salt wasting forms and incorrect sex of rearing in females with simple virilizing form. The diagnosis is reached by demonstrating high serum 17-hydroxyprogesterone (17OHP) levels. Preterm birth and neonatal illness can cause physiological elevation of 17OHP, thus complicating the diagnosis of CAH in the newborn period. Prenatal diagnosis and treatment with dexamethasone to prevent virilization of affected female fetuses is another area of controversy. The management of CAH is complicated by the need to use supraphysiologic doses of glucocorticoids to suppress adrenal androgen synthesis. In this review, the authors address pertinent issues related to the diagnosis and management of CAH in children.

Similar content being viewed by others
References
Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–88.
White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–91.
Maiti A, Chatterjee S. Congenital adrenal hyperplasia: An Indian experience. J Paediatr Child Health. 2011;47:883–7.
Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al; Endocrine Society. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–60.
Rama Devi AR, Naushad SM. Newborn screening in India. Indian J Pediatr. 2004;71:157–60.
Sharma S, Gupta DK. Male genitoplasty for 46 XX congenital adrenal hyperplasia patients presenting late and reared as males. Indian J Endocrinol Metab. 2012;16:935–8.
New MI, Lorenzen F, Lerner AJ, Kohn B, Oberfield SE, Pollack MS, et al. Genotyping steroid 21-hydroxylase deficiency: Hormonal reference data. J Clin Endocrinol Metab. 1983;57:320–6.
Dauber A, Kellogg M, Majzoub JA. Monitoring of therapy in congenital adrenal hyperplasia. Clin Chem. 2010;56:1245–51.
Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–93.
Janzen N, Riepe FG, Peter M, Sander S, Steuerwald U, Korsch E, et al. Neonatal screening: Identification of children with 11β-hydroxylase deficiency by second-tier testing. Horm Res Paediatr. 2012;77:195–9.
Kösel S, Burggraf S, Fingerhut R, Dörr HG, Roscher AA, Olgemöller B. Rapid second-tier molecular genetic analysis for congenital adrenal hyperplasia attributable to steroid 21-hydroxylase deficiency. Clin Chem. 2005;51:298–304.
Olgemöller B, Roscher AA, Liebl B, Fingerhut R. Screening for congenital adrenal hyperplasia: Adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab. 2003;88:5790–4.
Chennuri VS, Mithbawkar SM, Mokal RA, Desai MP. Serum 17 alpha hydroxyprogesterone in normal full term and preterm vs sick preterm and full term newborns in a tertiary hospital. Indian J Pediatr. 2013;80:21–5.
van der Kamp HJ, Wit JM. Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol. 2004;151:U71–5.
Chan CL, McFann K, Taylor L, Wright D, Zeitler PS, Barker JM. Congenital adrenal hyperplasia and the second newborn screen. J Pediatr. 2013;163:109–13el.
Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–72.
Mathur R, Menon PS, Kabra M, Goyal RK, Verma IC. Molecular characterization of mutations in Indian children with congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2001;14:27–35.
Hirvikoski T, Lindholm T, Lajic S, Nordenström A. Gender role behaviour in prenatally Dexamethasone-treated children at risk for congenital adrenal hyperplasia—A pilot study. Acta Paediatr. 2011;100:e112–9.
Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Wedell A, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92:542–8.
Meyer-Bahlburg HF, Dolezal C, Haggerty R, Silverman M, New MI. Cognitive outcome of offspring from dexamethasone-treated pregnancies at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167:103–10.
Witchel SF, Miller WL. Prenatal treatment of congenital adrenal hyperplasia-not standard of care. J Genet Couns. 2012;21:615–24.
New MI, Abraham M, Yuen T, Lekarev O. An update on prenatal diagnosis and treatment of congenital adrenal hyperplasia. Semin Reprod Med. 2012;30:396–9.
Joint LWPES/ESPE CAH Working Group. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab. 2002;87:4048–53.
Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: The ability to achieve normal growth. Pediatrics. 2000;106:767–73.
Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–36.
Kulshreshtha B, Eunice M, Ammini AC. Pubertal development among girls with classical congenital adrenal hyperplasia initiated on treatment at different ages. Indian J Endocrinol Metab. 2012;16:599–603.
Muthusamy K, Elamin MB, Smushkin G, Murad MH, Lampropulos JF, Elamin KB, et al. Clinical review: Adult height in patients with congenital adrenal hyperplasia: A systematic review and metaanalysis. J Clin Endocrinol Metab. 2010;95:4161–72.
Van der Kamp HJ, Otten BJ, Buitenweg N, De Muinck Keizer-Schrama SM, Oostdijk W, Jansen W, et al. Longitudinal analysis of growth and puberty in 21-hydroxylase deficiency patients. Arch Dis Child. 2002;87:139–44.
Muirhead S, Sellers EA, Guyda H, Canadian Pediatric Endocrine Group. Indicators of adult height outcome in classical 21-hydroxylase deficiency congenital adrenal hyperplasia. J Pediatr. 2002;141:247–52.
Stikkelbroeck NM, Van’t Hof-Grootenboer BA, Hermus AR, Otten BJ, Van’t Hof MA. Growth inhibition by glucocorticoid treatment in salt wasting 21-hydroxylase deficiency: In early infancy and (pre)puberty. J Clin Endocrinol Metab. 2003;88:3525–30.
Bonfig W, Schmidt H, Schwarz HP. Growth patterns in the first three years of life in children with classical congenital adrenal hyperplasia diagnosed by newborn screening and treated with low doses of hydrocortisone. Horm Res Paediatr. 2011;75:32–7.
Charmandari E, Brook CG, Hindmarsh PC. Why is management of patients with classical congenital adrenal hyperplasia more difficult at puberty? Arch Dis Child. 2002;86:266–9.
Bonfig W, Pozza SB, Schmidt H, Pagel P, Knorr D, Schwarz HP. Hydrocortisone dosing during puberty in patients with classical congenital adrenal hyperplasia: An evidence-based recommendation. J Clin Endocrinol Metab. 2009;94:3882–8.
Balsamo A, Cicognani A, Baldazzi L, Barbaro M, Maronio F, Gennari M, et al. CYP21 genotype, adult height, and pubertal development in 55 patients treated for 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2003;88:5680–8.
Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: Deceleration of growth velocity during puberty. J Clin Endocrinol Metab. 2007;92:1635–9.
Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: A re-evaluation of relative potency. J Pediatr. 2003;143:402–5.
Bajpai A, Pandey RM, Kabra M, Menon PS. Growth pattern and final height in 21-hydroxylase deficiency. Indian Pediatr. 2007;44:771–3.
Braga LH, Pippi Salle JL. Congenital adrenal hyperplasia: A critical appraisal of the evolution of feminizing genitoplasty and the controversies surrounding gender reassignment. Eur J Pediatr Surg. 2009;19:203–10.
Krege S, Walz KH, Hauffa BP, Körner I, Rübben H. Long-term follow-up of female patients with congenital adrenal hyperplasia from 21-hydroxylase deficiency, with special emphasis on the results of vaginoplasty. BJU Int. 2000;86:253–8.
Crouch NS, Liao LM, Woodhouse CR, Conway GS, Creighton SM. Sexual function and genital sensitivity following feminizing genitoplasty for congenital adrenal hyperplasia. J Urol. 2008;179:634–8.
Van Wyk JJ, Ritzen EM. The role of bilateral adrenalectomy in the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88:2993–8.
Deutschbein T, Unger N, Hauffa BP, Schaaf K, Mann K, Petersenn S. Monitoring medical treatment in adolescents and young adults with congenital adrenal hyperplasia: Utility of salivary 17α-hydroxyprogesterone day profiles. Exp Clin Endocrinol Diabetes. 2011;119:131–8.
Sarafoglou K, Himes JH, Lacey JM, Netzel BC, Singh RJ, Matern D. Comparison of multiple steroid concentrations in serum and dried blood spots throughout the day of patients with congenital adrenal hyperplasia. Horm Res Paediatr. 2011;75:19–25.
Lin-Su K, Harbison MD, Lekarev O, Vogiatzi MG, New MI. Final adult height in children with congenital adrenal hyperplasia treated with growth hormone. J Clin Endocrinol Metab. 2011;96:1710–7.
Merke DP, Keil MF, Jones JV, Fields J, Hill S, Cutler Jr GB. Flutamide, testolactone, and reduced hydrocortisone dose maintain normal growth velocity and bone maturation despite elevated androgen levels in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:1114–20.
Verma S, Vanryzin C, Sinaii N, Kim MS, Nieman LK, Ravindran S, et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2010;72:441–7.
Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94:3477–80.
Conflict of Interest
None.
Role of Funding Source
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, R., Seth, A. Congenital Adrenal Hyperplasia: Issues in Diagnosis and Treatment in Children. Indian J Pediatr 81, 178–185 (2014). https://doi.org/10.1007/s12098-013-1280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-013-1280-8