Abstract
Objective
To investigate the longitudinal changes in amino acid (AA) and acylcarnitine (AC) profiles of preterm neonates over the first 2 wk of life, and to detect any significant deviation from full term values that requires change of cut-off values used for detection of metabolic disorders in preterm neonates.
Methods
This observational analytical longitudinal study was conducted on 131 premature neonates (gestational age ranged from 27 to 36 wk) and 143 healthy full-term neonates. Dried blood spots were taken on the 5th and 14th postnatal day from the premature neonates and on day 5 from full term neonates for neonatal screening. Samples were analyzed for AA and AC using tandem mass spectrometer.
Results
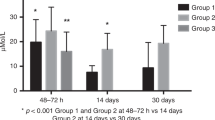
Most AA significantly decreased on day 14 compared to day 5 among preterm neonates (p < 0.05). The combined values of total carnitine (TC), total acylcarnitine (tAC) and short-chain acylcarnitines on day 5 among preterm neonates were statistically significantly higher compared to the day 14 sample (p 0.0001), whereas no statistically significant difference was found regarding the values of medium-, long-chain acylcarnitines, tAC/FC, and FC/TC (p > 0.05). The levels of AA of preterm neonates were statistically significantly higher than that of the controls (p < 0.05). The values of TC, tAC, short-, medium- and long-chain acylcarnitines, were significantly higher than those of the controls (p < 0.05). The reference ranges for preterm neonates were determined using the 1st and 99.9th percentiles.
Conclusions
AA and AC showed an age-related distribution of their concentrations. This underlines the importance of using appropriate reference values when working with a prematurely born population.

Similar content being viewed by others
References
Zytkovicz TH, Fitzgerald EF, Marsden D, et al. Tandem-mass spectrometric analysis of amino, organic, and fatty acid disorders in newborn dried blood spots: a two year summary from the New England Newborn Screening Program. Clin Chem. 2001;47:1945–55.
Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–817.
Antonucci R, Atzori L, Barberini L, Fanos V. Metabolomics: the “new clinical chemistry” for personalized neonatal medicine. Minerva Pediatr. 2010;62:145–8.
Cavedon CT, Bourdoux P, Mertens K, et al. Age-related variations in acylcarnitine and free carnitine concentrations measured by tandem mass spectrometry. Clin Chem. 2005;51:745–52.
Meyburg J, Schulze A, Kohlmueller D, et al. Acylcarnitine profiles of preterm infants over the first four weeks of life. Pediatr Res. 2002;52:720–3.
Meyburg J, Schulze A, Kohlmüller D, Linderkamp O, Mayatepek E. Postnatal changes in neonatal acylcarnitine profile. Pediatr Res. 2001;49:125–9.
Chace DH, Kalas TA. A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem. 2005;38:296–309.
Knapp RG, Miller MC. Describing the performance of a diagnostic test. Clinical epidemiology and biostatistics. Baltimore: Williams & Wilkins Harwal Pub. Co.; 1992. pp. 42.
World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects, the 59th WMA General Assembly, Seoul, South Korea; 2008.
Clark RH, Chace DH, Spitzer AR. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics. 2007;120:1286–96.
dos Santos SC, de Figueiredo CM, de Andrade SMO, Palhares DB. Plasma amino acids in preterm infants fed different human milk diets from a human milk bank. e-SPEN European e-J Clin Nutr Metabol. 2007;2:51–6.
Greene CL, Thomas JA, Goodman SI. Inborn errors of metabolism. In: Hay WW, Hayward A, Levin MJ, Sondheimer JM, eds. Current pediatric diagnosis & treatment. 16th ed. New York: Lange and McGraw-Hill Professional; 2003. pp. 986–1008.
Duran M. Amino acids. In: Blau N, Duran M, Gibson KM, eds. Laboratory guide to the methods in biochemical genetics. Berlin: Springer Press; 2008. pp. 53–90.
Balistreri WF. Immaturity of hepatic excretory function and the ontogeny of bile acid metabolism. J Pediatr Gastroenterol Nutr. 1983;2:S207–14.
Mirtallo J, Canada TW, Johnson D, et al. Task force for the revision of safe practices for parenteral nutrition. Safe practices for parenteral nutrition. JPEN J Parenter Enteral Nutr. 2004;28:S39–70.
ten Hoedt AE, van Kempen AA, Boelen A, et al. High incidence of hypermethioninaemia in a single neonatal intensive care unit detected by a newly introduced neonatal screening programme. J Inherit Metab Dis. 2007;30:978.
Misra PK, Awasthi S, Malik GK, Saksena PN, Agarwal S. Amino acid pattern in breast versus artificially fed babies. Indian J Pediatr. 1987;54:689–94.
Camelo Jr JS, Martinez FE, Goncalves AL, Monteiro JP, Jorge SM. Plasma amino acids in pregnancy, maternal intervillous space and preterm newborn infants. Braz J Med Biol Res. 2007;40:971–7.
Shenai JP, Borum PR. Tissue carnitine reserves of newborn infants. Pediatr Res. 1984;18:679–82.
Penn D, Ludwigs B, Schmidt SE, Pascu F. Effect of nutrition on tissue carnitine concentrations in infants of different gestational ages. Biol Neonate. 1985;47:130–5.
Shenai JP, Borum PR, Mohan P, Donlevy SC. Carnitine status at birth of newborn infants of varying gestation. Pediatr Res. 1983;17:579–82.
Cederblad G, Svenningsen N. Plasma carnitine and breast milk carnitine intake in premature infants. J Pediatr Gastroenterol Nutr. 1986;5:616–21.
Gong ZH, Tian GL, Wang YM. Bloodspot carnitine and acylcarnitine in newborn to adolescence: measured by tandem mass spectrometry. Zhonghua Er Ke Za Zhi. 2010;48:922–7.
Bonner CM, DeBrie KL, Hug G, Landrigan E, Taylor BJ. Effects of parenteral L-carnitine supplementation on fat metabolism and nutrition in premature neonates. J Pediatr. 1995;126:287–92.
Shortland GJ, Walter JH, Stroud C, Fleming PJ, Speidel BD, Marlow N. Randomised controlled trial of L-carnitine as a nutritional supplement in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F185–8.
Conflict of Interest
None.
Role of Funding Source
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandour, I., El Gayar, D., Amin, M. et al. Amino Acid and Acylcarnitine Profiles in Premature Neonates: A Pilot Study. Indian J Pediatr 80, 736–744 (2013). https://doi.org/10.1007/s12098-013-0980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-013-0980-4