Abstract
Objectives
No head-to-head trials had been performed to estimate the relative effectiveness of poly ADP-ribose polymerase inhibitor (PARPi) and androgen receptor signaling inhibitor (ARSi) in the first-line treatment for metastatic castration-resistant prostate cancer (mCRPC). We aimed to perform a systematic review and network meta-analysis to evaluate the comparative effectiveness of various systemic treatment agents for patients with mCRPC.
Methods
A comprehensive literature search was conducted for abstracts and full-text articles from the database’s inception through April 27, 2023. The study concentrated on assessing radiographic progression-free survival (rPFS) for both overall and homologous recombination repair mutation (HRRm) population, with overall survival (OS) as the secondary measure. Under the Bayesian framework, the overall effect was pooled using the fixed-effects model in base case analysis. Scenario analysis using restricted mean survival time (RMST) methods was performed to test the robustness of the results.
Results
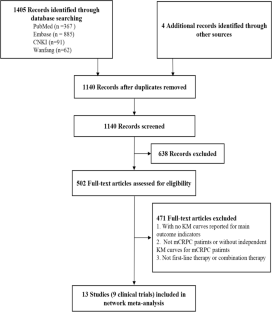
Nine studies with 6,830 patients and 8 unique treatment options were included. Network meta-analysis demonstrated that talazoparib in combination with enzalutamide (TALA + ENZA; overall population, hazard ratio [HR], 0.20; 95% credible interval [CrI]: 0.16–0.26; RMST, 3.51; 95% confidence interval [CI] 2.46–4.60; HRRm population, HR, 0.15; 95% CrI: 0.09–0.23; RMST, 4.14; 95% CI 2.84–5.39) was superior to other treatments in the first-line setting in terms of rPFS. The results of Bayesian framework and RMST models showed consistent efficacy ranks. When extrapolated to overall survival benefit, within the Bayesian framework, olaparib plus abiraterone acetate and prednisone (OLAP + AAP) achieved the highest OS benefit for the overall population, which was not statistically significant when compared to TALA + ENZA. However, TALA + ENZA achieved the highest OS benefit at 3 years by applying RMST.
Conclusions
We suggest that talazoparib in combination with enzalutamide is probably a preferred treatment agent for the overall population and HRRm patients with mCRPC. Given the limitations of network framework and the modeling assumptions undertaken to finalize the analyses, results should be cautiously interpreted.


Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AAP:
-
Abiraterone acetate plus prednisone
- ADT:
-
Androgen-deprivation therapy
- APA:
-
Apalutamide
- ARSi:
-
Androgen receptor signaling inhibitor
- BIC:
-
Bicalutamide
- BICR:
-
Blinded independent central review
- BSC:
-
Best support care
- BSP:
-
Bone sialoprotein
- CI:
-
Confidence interval
- CrI:
-
Credible interval
- DDR:
-
DNA damage response and repair
- ENZA:
-
Enzalutamide
- FDA:
-
Food and Drug Administration
- HR:
-
Hazard ratio
- HRRm:
-
Homologous recombination repair mutation
- HSP90:
-
Heat shock protein 90
- INV:
-
Investigator
- IPD:
-
Individual patient data
- KM:
-
Kaplan–Meier
- mCRPC:
-
Metastatic castration-resistant prostate cancer
- MMR:
-
Mismatch repair
- NCCN:
-
National Comprehensive Cancer Network
- NHEJ:
-
Non-homologous end-joining
- NMA:
-
Network meta-analysis
- NIRA:
-
Niraparib
- OLAP:
-
Olaparib
- OS:
-
Overall survival
- OSF:
-
Open Science Framework
- OPN:
-
Osteopontin
- PARPi:
-
Poly ADP-ribose polymerase inhibitor
- PH:
-
Proportional hazard
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROSPERO:
-
International Prospective Register of Systematic
- RCT:
-
Randomized controlled trial
- rPFS:
-
Radiographic progression-free survival
- SABR:
-
Stereotactic ablative radiotherapy
- TALA:
-
Talazoparib
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 CANCERS in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338.
Ma C, Ye D, Li C, Zhou F, Yao X, Zhang S, et al. Epidemiologic characteristics of prostate cancer and analysis of first-line endocrine therapy in advanced stage. Chin J Surg. 2008;12:921–5.
Zhu Y. Expert consensus on the diagnosis and treatment of denervation-resistant prostate cancer in China. Chin Surg Miscell J. 2016;54(7):481–4.
Attard G, Murphy L, Clarke NW, Sachdeva A, Jones C, et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023;24(5):443–56. https://doi.org/10.1016/S1470-2045(23)00148-1.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. https://doi.org/10.1056/NEJMoa041318.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53. https://doi.org/10.1056/NEJMoa1603144.
Shah S, Rachmat R, Enyioma S, Ghose A, Revythis A, et al. BRCA mutations in prostate cancer: assessment, implications and treatment considerations. Int J Mol Sci. 2021;22(23):12628. https://doi.org/10.3390/ijms222312628.
Boussios S, Rassy E, Shah S, Ioannidou E, Sheriff M, et al. Aberrations of DNA repair pathways in prostate cancer: a cornerstone of precision oncology. Expert Opin Ther Targets. 2021;25(5):329–33. https://doi.org/10.1080/14728222.2021.1951226.
Yang S, Liu X. Progress of drug therapy for metastatic denervation-resistant prostate cancer. J Med Res. 2022;51(11):4–9.
Agarwal N, Azad AA, Carles J, Fay AP, Matsubara N, Heinrich D, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;S0140–6736(23):01055–63. https://doi.org/10.1016/S0140-6736(23)01055-3.
Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M,Shore ND, Procopio G, et al. (2023) Final pre-specified overall survival in PROpel: abiraterone and olaparib versus abiraterone and placebo as first-line therapy for metastatic castration-resistant prostate cancer. ASCO GU. https://ascopubs.org/doi/abs/https://doi.org/10.1200/JCO.2023.41.6_suppl.LBA16.
Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(18):3339–51. https://doi.org/10.1200/JCO.22.01649.
Wei Y, Zhang T, He Y, Efstathiou E, Attard G, Olmos D, et al. Preliminary efficacy and safety study of fluzoparib in the treatment of metastatic desmoplasia-resistant prostate cancer. Chin J Cancer. 2021;31(07):561–6. https://doi.org/10.19401/j.cnki.1007-3639.2021.07.001.
Ghose A, Moschetta M, Pappas-Gogos G, Sheriff M, Boussios S. Genetic aberrations of DNA repair pathways in prostate cancer: translation to the clinic. Int J Mol Sci. 2021;22(18):9783. https://doi.org/10.3390/ijms22189783.
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60. https://doi.org/10.1016/S1470-2045(14)71205-7.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33. https://doi.org/10.1056/NEJMoa1405095.
Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22(11):1541–59. https://doi.org/10.1016/S1470-2045(21)00402-2.
Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE Trial. J Clin Oncol. 2016;34(18):2098–106. https://doi.org/10.1200/JCO.2015.64.9285.
Higano CS, Cheng HH. Poly-ADP ribose polymerase inhibitor and androgen receptor signaling inhibitor for all comers for first-line treatment of metastatic castration-resistant prostate cancer: is gene sequencing out? Curr Opin Urol. 2023;33(5):396–403. https://doi.org/10.1097/MOU.0000000000001114.
NCCN (2022) The NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer(version 1.2023) [EB/OL]. http://www.nccn.org.
National Health Commission of the People's Republic of China. Guidelines for the diagnosis and treatment of prostate cancer [EB/OL]. http://www.nhc.gov.cn/yzygj/s7659/202204/a0e67177df1f439898683e1333957c74/files/64eb7728ee494e299a77846fff09840e.pdf.
Pan J, Zhao J, Ni X, Gan H, Wei Y, Wu J, et al. The prevalence and prognosis of next-generation therapeutic targets in metastatic castration-resistant prostate cancer. Mol Oncol. 2022;16(22):4011–22.
Saxby H, Boussios S, Mikropoulos C. Androgen receptor gene pathway upregulation and radiation resistance in oligometastatic prostate cancer. Int J Mol Sci. 2022;23(9):4786. https://doi.org/10.3390/ijms23094786.
Saxby H, Mikropoulos C, Boussios S. An update on the prognostic and predictive serum biomarkers in metastatic prostate cancer. Diagnostics (Basel). 2020;10(8):549. https://doi.org/10.3390/diagnostics10080549.
Fang M, Nakazawa M, Antonarakis ES, Li C. Efficacy of Abiraterone and enzalutamide in pre- and postdocetaxel castration-resistant prostate cancer: a trial-level meta-analysis. Prostate Cancer. 2017;2017:8560827. https://doi.org/10.1155/2017/8560827.
Iannantuono GM, Chandran E, Floudas CS, et al. Efficacy and safety of PARP inhibitors in metastatic castration-resistant prostate cancer: a systematic review and meta-analysis of clinical trials. Cancer Treat Rev. 2023;120: 102623. https://doi.org/10.1016/j.ctrv.2023.102623.
Hutton B, Salanti G, Caldwell DM, Choo-Wosoba H, Butera G, Roselli M, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385.
Ai J (2023) Comparative effectiveness of poly ADP-ribose polymerase inhibitor and androgen receptor signaling inhibitor in the first-line treatment for metastatic castration-resistant prostate cancer: a network meta-analysis. Accessed Sep 7, 2023. https://osf.io/axpuk.
Ai J. Comparative effectiveness of poly ADP-ribose polymerase inhibitor and androgen receptor signaling inhibitor in the first-line treatment for metastatic castration-resistant prostate cancer: a network meta-analysis. Accessed Sep 26, 2023. https://www.crd.york.ac.uk/prospero
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Chen J, Zhang Y, Zhang X, Zhao J, Ni Y, Zhu S, et al. Comparison of systemic treatments for metastatic castration-resistant prostate cancer after docetaxel failure: a systematic review and network meta-analysis. Front Pharmacol. 2022;12:789319. https://doi.org/10.3389/fphar.2021.789319.
Pak K, Uno H, Kim DH, Tian L, Kane RC, Takeuchi M, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3(12):1692–6. https://doi.org/10.1001/jamaoncol.2017.2797.
Lombardi P, Filetti M, Falcone R, Di Bidino R, Iacovelli R, Ciccarese C, et al. New first-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Cancer Treat Rev. 2022;106:102377. https://doi.org/10.1016/j.ctrv.2022.102377.
Fisher D (2019) IPDMETAN: Stata module for performing two-stage IPD meta-analysis. Stata Software Components. Available at: https://econpapers.repec.org/software/bocbocode/S457785.htm.
Therneau GTM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. https://doi.org/10.1186/1471-2288-12-9.
Petit C, Blanchard P, Pignon JP, Lueza B. Individual patient data network meta-analysis using either restricted mean survival time difference or hazard ratios: is there a difference? A case study on locoregionally advanced nasopharyngeal carcinomas. Syst Rev. 2019;8(1):96. https://doi.org/10.1186/s13643-019-0984-x.
Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94.
Pu YS, Ahn H, Han W, Huang SP, Wu HC, Ma L, et al. Enzalutamide in chemotherapy-naïve metastatic castration-resistant prostate cancer: an Asian multiregional. Random Study Adv Ther. 2022;39(6):2641–56. https://doi.org/10.1007/s12325-022-02140-2.
Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151–4. https://doi.org/10.1016/j.eururo.2016.07.032.
Oya M, Armstrong AJ, Thiery-Vuillemin A, Shore N, Procopio G, Arslan C, et al. (2022) Biomarker analysis and updated results from the Phase III PROpel trial of abiraterone (abi) and olaparib (ola) vs abi and placebo (pbo) as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). ESMO 2022. https://d27mnwjqm5ztsa.cloudfront.net/4d4d150a-bf20-4c76-945a-ce2a670c3c99/031898bb-a199-4394-b7fa-aa1c6f7a6a2a/031898bb-a199-4394-b7fa-aa1c6f7a6a2a_source__v.pdf.
Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–25. https://doi.org/10.1016/j.eururo.2014.02.056.
Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153–63. https://doi.org/10.1016/S1470-2045(15)00518-5.
Armstrong AJ, Lin P, Tombal B, Saad F, Higano CS, Joshua AM, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL Trial. Eur Urol. 2020;78(3):347–57. https://doi.org/10.1016/j.eururo.2020.04.061.
Fizazi K, Azad A, Matsubara N, Carles J, Fay AP, Giorgi UD, et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) harboring homologous recombination repair (HRR) gene alterations. https://doi.org/10.1200/JCO.2023.41.16_suppl.5004.
Authors' Contributions
JA and TZ had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the paper has been performed by the authors, unless clearly specified in the text. Conception and design: JA, TZ. Acquisition, analysis, or interpretation of data: JA, LJ, XW. Drafting of the manuscript: JA, XH. Critical revision of the manuscript for important intellectual content: XY, JJ. Statistical analysis: JA, LJ, XH. Obtained funding: TZ. Administrative, technical, or material support: JJ, TZ. Supervision: TZ.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 72274079). Role of the sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript is based entirely on the analysis of previously published data. As such, this study does not involve any direct research with human participants or animals, and therefore, it does not require ethical approval.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ai, J., Jian, L., Wen, X. et al. Comparative effectiveness of first-line systemic treatments for metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03506-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03506-4




