Abstract
Purpose
Progression after first-line immunochemotherapy (ICT) for recurrent or metastatic nasopharyngeal carcinoma (R/M NPC) is a clinical concern due to subsequent limited treatment options. This study firstly predicted the progress outcome.
Methods
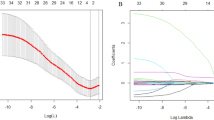
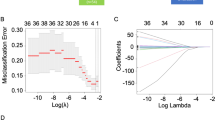
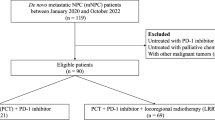
A cohort of 186 R/M NPC cases that received first-line ICT was included for developing a Cox regression model for progression-free survival (PFS) and risk stratification, which was verified by cross-validation. Discrimination and calibration were evaluated. Progression sites in risk groups was shown with a Sankey diagram.
Results
Baseline predictors including liver metastasis, trend of plasma Epstein–Barr virus DNA copies, lymphocyte-to-monocyte ratio, and level of platelet and lactate dehydrogenase were identified for model construction, which stratify the cohort into low, middle, and high-risk groups. The overall concordance index (C-index) was 0.67 (95% CI 0.62–0.73). The area under the curve (AUC) was 0.68 (95% CI 0.60–0.76), 0.74 (95% CI 0.66–0.82), 0.75 (95% CI 0.65–0.84) at predicting 12, 18, and 24 months PFS, indicating a moderate accuracy. Cross-validation showed the model performance was robust. Compared with the low-risk group (median PFS: 24.4 months, 95% CI 18.4 months to not reached), the high-risk group (median PFS: 7.1 months, 95% CI 6.4–10.1 months; hazard risk: 7.4, 95% CI 4.4–12.4, p < 0.001) progressed with more liver metastasis after ICT resistance.
Conclusion
It was the first study that described the risk factors and progression characteristics in R/M NPC patients who received first-line ICT, investigating the progression patterns, which was helpful to identify patients with different risks and help guide personalized interventions.






Similar content being viewed by others
Data availability
The dataset used and analysed during the current study is available from the corresponding author on reasonable request.
Change history
28 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12094-024-03389-5
References
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80.
Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie A, Mendenhall WM, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019;79: 101890.
Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883–92.
Hong S, Zhang Y, Yu G, Peng P, Peng J, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as first-line therapy for recurrent or metastatic nasopharyngeal carcinoma: final overall survival analysis of gem20110714 phase iii study. J Clin Oncol. 2021;39:3273–82.
Johnson D, Ma BBY. Targeting the PD-1/ PD-L1 interaction in nasopharyngeal carcinoma. Oral Oncol. 2021;113: 105127.
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22:1162–74.
Yang Y, Pan J, Wang H, Zhao Y, Qu S, Chen N, et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: A multicenter phase 3 trial (RATIONALE-309). Cancer Cell. 2023.
Adkins DR, Haddad RI. Clinical trial data of anti-pd-1/pd-l1 therapy for recurrent or metastatic nasopharyngeal carcinoma: a review. Cancer Treat Rev. 2022;109: 102428.
Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37:443–55.
Huang H, Li S, Tang Q, Zhu G. Metabolic reprogramming and immune evasion in nasopharyngeal carcinoma. Front Immunol. 2021;12: 680955.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Yang Y, Zhou T, Chen X, Li J, Pan J, He X, et al. Efficacy, safety, and biomarker analysis of camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study). J Immunother Cancer. 2021;9.
Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase ii clinical trial (POLARIS-02). J Clin Oncol. 2021;39:704–12.
Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol. 2018;36:1412–8.
Ma Y, Fang W, Zhang Y, Yang Y, Hong S, Zhao Y, et al. A Phase I/II Open-Label Study of Nivolumab in Previously Treated Advanced or Recurrent Nasopharyngeal Carcinoma and Other Solid Tumors. Oncologist. 2019;24:891-e431.
Even C, Wang HM, Li SH, Ngan RK, Dechaphunkul A, Zhang L, et al. Phase II, Randomized Study of Spartalizumab (PDR001), an Anti-PD-1 Antibody, versus Chemotherapy in Patients with Recurrent/Metastatic Nasopharyngeal Cancer. Clin Cancer Res. 2021;27:6413–23.
Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-depth mining of clinical data: the construction of clinical prediction model with R. Ann Transl Med. 2019;7:796.
Xu JY, Wei XL, Ren C, Zhang Y, Hu YFtwo-sided, Li JY, et al. Association of Plasma Epstein-Barr Virus DNA With Outcomes for Patients With Recurrent or Metastatic Nasopharyngeal Carcinoma Receiving Anti-Programmed Cell Death 1 Immunotherapy. JAMA Netw Open. 2022;5:e220587.
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27:1536–43.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–64.
Lee JC, Green MD, Huppert LA, Chow C, Pierce RH, Daud AI. The Liver-Immunity Nexus and Cancer Immunotherapy. Clin Cancer Res. 2022;28:5–12.
Huemer F, Lang D, Westphal T, Gampenrieder SP, Hutarew G, Weiss L, et al. Baseline Absolute Lymphocyte Count and ECOG Performance Score Are Associated with Survival in Advanced Non-Small Cell Lung Cancer Undergoing PD-1/PD-L1 Blockade. J Clin Med. 2019;8.
Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125.
Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell. 2018;33:965–83.
Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–70.
Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201-18.e9.
Acknowledgements
We sincerely thank Dr. Jialin Wu for providing technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethics approval
This retrospective study was approved by Sun Yat-sen University Cancer Center (IRB-approved number, B2022-797).
Informed consent
All clinical information was anonymous, and the requirement for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to include Table 1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, D., Zhang, Y., He, S. et al. Predictive progression outcomes and risk stratification in patients with recurrent or metastatic nasopharyngeal carcinoma who received first-line immunochemotherapy. Clin Transl Oncol 26, 1209–1219 (2024). https://doi.org/10.1007/s12094-023-03344-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03344-w