Abstract
Purpose
To study the clinicopathological variables connected with disease-free survival (DFS) as well as overall survival (OS) in patients who are ER-positive or HER2-negative and to propose nomograms for predicting individual risk.
Methods
In this investigation, we examined 585 (development cohort) and 291 (external validation) ER-positive, HER2-negative breast cancer patients from January 2010 to January 2014. From January 2010 to December 2014, we retrospectively reviewed and analyzed 291 (external validation) and 585 (development cohort) HER2-negative, ER-positive breast cancer patients. Cox regression analysis, both multivariate and univariate, confirmed the independence indicators for OS and DFS.
Results
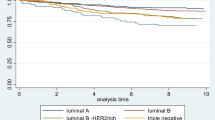
Using cox regression analysis, both multivariate and univariate, the following variables were combined to predict the DFS of development cohort: pathological stage (HR = 1.391; 95% CI = 1.043–1.855; P value = 0.025), luminal parting (HR = 1.836; 95% CI = 1.142–2.952; P value = .012), and clinical stage (HR = 1.879; 95% CI = 1.102–3.203; P value = 0.021). Endocrine therapy (HR = 3.655; 95% CI = 1.084–12.324; P value = 0.037) and clinical stage (HR = 6.792; 95% CI = 1.672–28.345; P value = 0.009) were chosen as predictors of OS. Furthermore, we generated RS-OS and RS-DFS. According to the findings of Kaplan–Meier curves, patients who are classified as having a low risk have considerably longer DFS and OS durations than patients who are classified as having a high risk.
Conclusion
To generate nomograms that predicted DFS and OS, independent predictors of DFS in ER-positive/HER2-negative breast cancer patients were chosen. The nomograms successfully stratified patients into prognostic categories and worked well in both internal validation and external validation.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Fan S, Yang Z, Ke Z, Huang K, Liu N, Fang X, et al. Downregulation of the long non-coding RNA TUG1 is associated with cell proliferation, migration, and invasion in breast cancer. Biomed Pharmacother. 2017;95:1636–43.
Johnston SR. Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin Cancer Res. 2006;12(3 Pt 2):1061s–8s.
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, ESMO Guidelines Working Group. ESMO Guidelines Working Group Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11-9.
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–95.
Woods JF, De Marchi JA, Lowery AJ, Hill AD. Validation of a nomogram predicting sentinel lymph node status in melanoma in an Irish population. Ir J Med Sci. 2015;184:769–73.
Cao J, Yuan P, Wang L, Wang Y, Ma H, Yuan X, et al. Clinical nomogram for predicting survival of esophageal cancer patients after esophagectomy. Sci Rep. 2016;6:26684.
Sun W, Jiang YZ, Liu YR, Ma D, Shao ZM. Nomograms to estimate long-term overall survival and breast cancer-specific survival of patients with luminal breast cancer. Oncotarget. 2016;7:20496–506.
Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: Toward improving individualized cancer care. Gynecol Oncol. 2010;116:399–403.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70.
Weiser MR, Landmann RG, Kattan MW, Gonen M, Shia J, Chou J, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–5.
Gondo OT, Riu Hamada M, Gondo T, Hamada R. Nomogram as predictive model in clinical practice. Gan To Kagaku Ryoho. 2009;6:901–6.
Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834–40.
Albert JM, Liu DD, Shen Y, Pan IW, Shih YC, Hoffman KE, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30:2837–43.
Mariani P, Dureau S, Savignoni A, Rouic LL, Levy-Gabriel C, Piperno-Neumann S, et al. Development of a prognostic nomogram for liver metastasis of uveal melanoma patients selected by liver MRI. Cancers (Basel). 2019;11:E863.
Author information
Authors and Affiliations
Contributions
XS conceived and designed the study, and drafted the manuscript. PW, RF, and MC collected, analyzed, and interpreted the experimental data. EL, XW, ZL, SL, and JL revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of The First Affiliated Hospital of Shantou University Medical College and conducted in accordance with the ethical standards.
Informed consent
Subjects signed the informed consent.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, X., Wang, P., Feng, R. et al. Prognostic model of ER-positive, HER2-negative breast cancer predicted by clinically relevant indicators. Clin Transl Oncol 26, 389–397 (2024). https://doi.org/10.1007/s12094-023-03316-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03316-0