Abstract
Introduction
Systemic therapy of patients with metastatic renal cell carcinoma (mRCC) has improved in the past years, with the advent of new immunotherapy-based combinations as a standard treatment option for first-line therapy. Nevertheless, particularly in good-risk patients by IMDC criteria, tyrosine-kinase inhibitors (TKI) may remain as an option for some patients. We reviewed our experience with TKI as first-line therapy for mRCC patients, trying to identify subgroups of patients that may still benefit from this strategy.
Material and methods
All patients with mRCC treated with first-line TKI, and adequate follow-up, in University Hospital La Paz (Madrid, Spain) between 2007 and 2020 were analyzed. Patients treated inside a clinical trial were excluded from this analysis.
Results
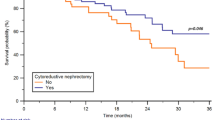
A total of 90 patients treated with first-line TKI were included. Regarding IMDC criteria, 33 patients (36.7%) were good-risk, 41 patients (45.5%) intermediate-risk, and 16 patients (17.8%) poor-risk. With a median follow-up of 49 months, the median overall survival (OS) for good, intermediate, and poor-risk patients was 54, 24, and 16 months (p = 0.004). When intermediate-risk was divided into patients with 1 or 2 risk factors, differences in OS were also statistically significant: patients with 1 risk factor had a median OS of 33 months, while patients with 2 risk factors had a median OS of 16 months, the same as poor-risk patients (p = 0.003). In the multivariate analysis, trying to find out which of the IMDC factors had a more remarkable weight in the prognosis of the patients, both ECOG and hemoglobin levels by themselves were significantly associated with OS.
Conclusion
In our group of patients, survival outcomes were different among patients with intermediate-risk with 1 or 2 risk factors by IMDC criteria. These could help select patients that may benefit from first-line treatment with a TKI, particularly in settings with difficult access to novel therapies, such as immunotherapy-based combinations.


Similar content being viewed by others
Data availability
Data available upon request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. 2020. https://gco.iarc.fr/today. Accessed 23 Nov 2022.
Sankin A, Hakimi AA, Hsieh JJ, Molina AM. Metastatic non-clear cell renal cell carcinoma: an evidence-based review of current treatment strategies. Front Oncol. 2015;5:67.
Surveillance epidemiology and end results database. Cancer of the kidney and renal pelvis—SEER 18, relative survival by stage (2010–2017). 2021.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: the 2022 update. Eur Urol. 2022;82:399–410.
Rini B, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–41.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300.
Rodríguez-Lescure A, de la Peña FA, Aranda E, Calvo A, Felip E, Garrido P, et al. Study of the Spanish Society of Medical Oncology (SEOM) on the access to oncology drugs and predictive biomarkers in Spain. Clin Transl Oncol. 2020;22:2253–63.
Rathmell WK, Rumble RB, van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–95.
Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38:803–17.
Mattila PO, Ahmad R, Hassan SS, Babar Z. Availability, affordability, access, and pricing of anti-cancer medicines in low- and middle-income countries: a systematic review of the literature. Front Pub Health. 2021;9:628744.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31.
Author information
Authors and Affiliations
Contributions
JM, ÁP: conceptualization, data curation, methodology, investigation, writing—original draft, writing—review and editing. AP: conceptualization, data curation, methodology, investigation, writing—original draft. MÁ-M, PG-P, AA, EG, LT, ÁG, EE: data curation, methodology, investigation.
Corresponding author
Ethics declarations
Conflict of interest
Álvaro Pinto: Speaker fees—advisory board: Janssen, Astellas, Pfizer, GSK, Novartis, Ipsen, Bristol-Myers-Squibb, MSD, Eisai, Bayer. Research grants: Pfizer, Bristol-Myers-Squibb. Ana Pertejo: Speaker fees—advisory board: Janssen, Astellas. The rest of the authors do not have relevant conflicts of interest to disclose.
Ethical approval (Research involving human participants and/or animals)
The study was approved by the Ethics Committee of Hospital Universitario La Paz, project PI-3310. This was a retrospective, non-interventional study, so there was no formal sample processing or research involving humans.
Informed consent
Informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pinto, Á., Miranda, J., Pertejo, A. et al. Different outcomes among patients with intermediate-risk metastastic renal cell carcinoma treated with first-line tyrosine-kinase inhibitors. Clin Transl Oncol 26, 532–537 (2024). https://doi.org/10.1007/s12094-023-03274-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03274-7