Abstract
Purpose
This study intends to investigate the possible molecular mechanism of immune response and tumorigenesis in ovarian cancer cells, mediated by sirtuin 1 (SIRT1)-containing extracellular vesicles (EVs) derived from cancer-associated adipocytes (CAAs) (CAA-EVs).
Methods
Differentially expressed genes in EVs from CAAs were screened by RNA transcriptome sequencing, and the downstream pathway was predicted in silico. The binding between SIRT1 and CD24 was investigated by luciferase activity and ChIP-PCR assays. EVs were extracted from human ovarian cancer tissue-isolated CAAs, and the internalization of CCA-EVs by ovarian cancer cells was characterized. The ovarian cancer cell line was injected into mice to establish an animal model. Flow cytometry was performed to analyze the proportions of M1 and M2 macrophages, CD8+ T, T-reg, and CD4+ T cells. TUNEL staining was used to detect cell apoptosis in the mouse tumor tissues. ELISA detection was performed on immune-related factors in the serum of mice.
Results
CAA-EVs could deliver SIRT1 to ovarian cancer cells, thereby affecting the immune response of ovarian cancer cells in vitro and promoting tumorigenesis in vivo. SIRT1 could transcriptionally activate the expression of CD24, and CD24 could up-regulate Siglec-10 expression. CAA-EVs-SIRT1 activated the CD24/Siglec-10 axis and promoted CD8+ T cell apoptosis, thereby promoting tumorigenesis in mice.
Conclusion
CAA-EVs-mediated transfer of SIRT1 regulates the CD24/Siglec-10 axis to curb immune response and promote tumorigenesis of ovarian cancer cells.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Maoz A, Matsuo K, Ciccone MA, Matsuzaki S, Klar M, Roman LD, et al. Molecular pathways and targeted therapies for malignant ovarian germ cell tumors and sex cord-stromal tumors: a contemporary review. Cancers (Basel). 2020;12:1398.
Zilovic D, Ciurliene R, Sabaliauskaite R, Jarmalaite S. Future screening prospects for ovarian cancer. Cancers (Basel). 2021;13:3840.
Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28:61–5.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53.
Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med. 2007;204:619–31.
Pekalski ML, Garcia AR, Ferreira RC, Rainbow DB, Smyth DJ, Mashar M, et al. Neonatal and adult recent thymic emigrants produce IL-8 and express complement receptors CR1 and CR2. JCI Insight. 2017. https://doi.org/10.1172/jci.insight.9373910.1172/jci.insight.93739.
Starzer AM, Preusser M, Berghoff AS. Immune escape mechanisms and therapeutic approaches in cancer: the cancer-immunity cycle. Ther Adv Med Oncol. 2022;14:17588359221096220.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Munteanu R, Onaciu A, Moldovan C, Zimta AA, Gulei D, Paradiso AV, et al. Adipocyte-based cell therapy in oncology the role of cancer-associated adipocytes and their reinterpretation as delivery platforms. Pharmaceutics. 2020;12:402.
Tian W, Lei N, Zhou J, Chen M, Guo R, Qin B, et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022;13:64.
Shen P, Deng X, Chen Z, Ba X, Qin K, Huang Y, et al. SIRT1: a potential therapeutic target in autoimmune diseases. Front Immunol. 2021;12:779177.
Lin Z, Fang D. The roles of SIRT1 in Cancer. Genes Cancer. 2013;4:97–104.
Zhang X, Chen J, Sun L, Xu Y. SIRT1 deacetylates KLF4 to activate Claudin-5 transcription in ovarian cancer cells. J Cell Biochem. 2018;119:2418–26.
Duan J, Yin M, Shao Y, Zheng J, Nie S. Puerarin induces platinum-resistant epithelial ovarian cancer cell apoptosis by targeting SIRT1. J Int Med Res. 2021;49:3000605211040762.
Wang TW, Chern E, Hsu CW, Tseng KC, Chao HM. SIRT1-mediated expression of CD24 and epigenetic suppression of novel tumor suppressor miR-1185-1 increases colorectal cancer stemness. Cancer Res. 2020;80:5257–69.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–6.
Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66.
Abram CL, Lowell CA. Shp1 function in myeloid cells. J Leukoc Biol. 2017;102:657–75.
Li Y, Zhou J, Zhuo Q, Zhang J, Xie J, Han S, et al. Malignant ascite-derived extracellular vesicles inhibit T cell activity by upregulating Siglec-10 expression. Cancer Manag Res. 2019;11:7123–34.
Lee J, Hong BS, Ryu HS, Lee HB, Lee M, Park IA, et al. Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism. PLoS ONE. 2017;12:e0174126.
Dai X, Xie Y, Dong M. Cancer-associated fibroblasts derived extracellular vesicles promote angiogenesis of colorectal adenocarcinoma cells through miR-135b-5p/FOXO1 axis. Cancer Biol Ther. 2022;23:76–88.
Pan D, Chen J, Feng C, Wu W, Wang Y, Tong J, et al. Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int J Mol Sci. 2019;20:3213.
Gu YY, Yu J, Zhang JF, Wang C. Suppressing the secretion of exosomal miR-19b by gw4869 could regulate oxaliplatin sensitivity in colorectal cancer. Neoplasma. 2019;66:39–45.
Fang X, Xu X, Lin X, Liu R. Downregulated spinal IRF8 and BDNF in NAC are involved in neuropathic pain-induced depression relief via pulsed radiofrequency on dorsal root ganglion in rat SNI model. Brain Res Bull. 2019;146:192–200.
Qi G, Zhang C, Ma H, Li Y, Peng J, Chen J, et al. CDCA8, targeted by MYBL2, promotes malignant progression and olaparib insensitivity in ovarian cancer. Am J Cancer Res. 2021;11:389–415.
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, et al. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol Res. 2018;6:1578–92.
Zheng J, Mao Y, Dong P, Huang Z, Yu F. Long noncoding RNA HOTTIP mediates SRF expression through sponging miR-150 in hepatic stellate cells. J Cell Mol Med. 2019;23:1572–80.
Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632.
Chen WX, Wang DD, Zhu B, Zhu YZ, Zheng L, Feng ZQ, et al. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging (Albany NY). 2021;13:10415–30.
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–20.
Guo JC, Yang YJ, Zheng JF, Zhang JQ, Guo M, Yang X, et al. Silencing of long noncoding RNA HOXA11-AS inhibits the Wnt signaling pathway via the upregulation of HOXA11 and thereby inhibits the proliferation, invasion, and self-renewal of hepatocellular carcinoma stem cells. Exp Mol Med. 2019;51:1–20.
Mufazalov IA, Regen T, Schelmbauer C, Kuschmann J, Muratova AM, Nikolaev A, et al. Generation of a Novel T Cell Specific Interleukin-1 Receptor Type 1 Conditional Knock Out Mouse Reveals Intrinsic Defects in Survival, Expansion and Cytokine Production of CD4 T Cells. PLoS ONE. 2016;11:e0161505.
Qu QX, Xie F, Huang Q, Zhang XG. Membranous and cytoplasmic expression of PD-L1 in ovarian cancer cells. Cell Physiol Biochem. 2017;43:1893–906.
Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158.
Yu PN, Yan MD, Lai HC, Huang RL, Chou YC, Lin WC, et al. Downregulation of miR-29 contributes to cisplatin resistance of ovarian cancer cells. Int J Cancer. 2014;134:542–51.
Salem M, Shan Y, Bernaudo S, Peng C. miR-590–3p Targets Cyclin G2 and FOXO3 to Promote Ovarian Cancer Cell Proliferation, Invasion, and Spheroid Formation. Int J Mol Sci. 1810;2019:20.
Zhu D, Yuan D, Guo R, Zhang L, Guo T, Zhao Y, et al. Overexpression of miR-148a inhibits viability and invasion of ovarian cancer OVCAR3 cells by targeting FOXO3. Oncol Lett. 2019;18:402–10.
Prantner AM, Yin C, Kamat K, Sharma K, Lowenthal AC, Madrid PB, et al. Molecular Imaging of Mesothelin-Expressing Ovarian Cancer with a Human and Mouse Cross-Reactive Nanobody. Mol Pharm. 2018;15:1403–11.
Chen F, Xu Y, Chen Y, Shan S. TIGIT enhances CD4(+) regulatory T-cell response and mediates immune suppression in a murine ovarian cancer model. Cancer Med. 2020;9:3584–91.
Fang Y, Tian Y, Huang Q, Wan Y, Xu L, Wang W, et al. Deficiency of TREK-1 potassium channel exacerbates blood-brain barrier damage and neuroinflammation after intracerebral hemorrhage in mice. J Neuroinflammation. 2019;16:96.
Frazzi R. SIRT1 in secretory organ cancer. Front Endocrinol (Lausanne). 2018;9:569.
Bose S, Saha P, Chatterjee B, Srivastava AK. Chemokines driven ovarian cancer progression, metastasis and chemoresistance: Potential pharmacological targets for cancer therapy. Semin Cancer Biol. 2022;86:568–79.
Arneth B. Tumor Microenvironment. Medicina (Kaunas). 2019;56:15.
Mukherjee A, Bilecz AJ, Lengyel E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev. 2022;41:575–87.
Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12:95.
Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55.
Czystowska-Kuzmicz M, Sosnowska A, Nowis D, Ramji K, Szajnik M, Chlebowska-Tuz J, et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat Commun. 2019;10:3000.
Liu ZM, Wang YB, Yuan XH. Exosomes from murine-derived GL26 cells promote glioblastoma tumor growth by reducing number and function of CD8+T cells. Asian Pac J Cancer Prev. 2013;14:309–14.
Maybruck BT, Pfannenstiel LW, Diaz-Montero M, Gastman BR. Tumor-derived exosomes induce CD8(+) T cell suppressors. J Immunother Cancer. 2017;5:65.
Roy S, Das A, Vernekar M, Mandal S, Chatterjee N. Understanding the correlation between metabolic regulator SIRT1 and exosomes with CA-125 in ovarian cancer: A clinicopathological study. Biomed Res Int. 2022;2022:5346091.
Wang Z, Yan Y, Lou Y, Huang X, Liu L, Weng Z, et al. Diallyl trisulfide alleviates chemotherapy sensitivity of ovarian cancer via the AMPK/SIRT1/PGC1alpha pathway. Cancer Sci. 2023;114:357–69.
Qin J, Liu Y, Lu Y, Liu M, Li M, Li J, et al. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci Rep. 2017;7:10592.
Song S, Tang H, Quan W, Shang A, Ling C. Estradiol initiates the immune escape of non-small cell lung cancer cells via ERbeta/SIRT1/FOXO3a/PD-L1 axis. Int Immunopharmacol. 2022;107: 108629.
Sammar M, Siwetz M, Meiri H, Fleming V, Altevogt P, Huppertz B. Expression of CD24 and Siglec-10 in first trimester placenta: implications for immune tolerance at the fetal-maternal interface. Histochem Cell Biol. 2017;147:565–74.
Funding
This study was supported by Medical Key Supporting Project of Suzhou City (No. SZFCXK202142); Key Projects of Medical and Health Science and Technology Plan of Suzhou Hi-tech Zone (No. 2020Z008) and Suzhou Science and Technology Development Plan (No. SKJYD2021058).
Author information
Authors and Affiliations
Contributions
QZ, XD, JZ and YL designed the study. WD, XD and DG collated the data, designed and developed the database, carried out data analyses and produced the initial draft of the manuscript. QZ and XD contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interests to declare.
Ethical approval
Our study was approved by the Clinical Ethics Committee and all tissue donors were required to sign a written informed consent.
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2023_3240_MOESM1_ESM.eps
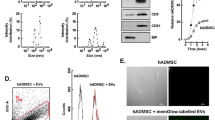
Supplementary file1 Fig. S1 Extraction and identification of normal-EVs and CAA-EVs. A: TEM to identify the structure of normal-EVs and CAA-EVs; B: Nanoparticle size analysis to detect the diameter size of normal-EVs and CAA-EVs; C: Western blot to detect the protein expression of TSG101, CD63, CD81, and GRP94 in normal adipocytes and in CAAs and their EVs. Cell experiments were repeated three times (EPS 4133 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Q., Du, X., Zhang, J. et al. Delivery of SIRT1 by cancer-associated adipocyte-derived extracellular vesicles regulates immune response and tumorigenesis of ovarian cancer cells. Clin Transl Oncol 26, 190–203 (2024). https://doi.org/10.1007/s12094-023-03240-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03240-3