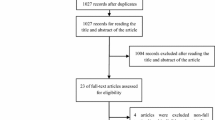
Abstract
Aim
To evaluate the methodological quality of clinical practice guidelines (CPGs) on treatments for non-small cell lung cancer (NSCLC).
Methods
We searched MEDLINE, CPG developer websites, lung cancer societies, and oncology organizations to identify CPGs providing recommendations on treatments for NSCLC. The methodological quality for each CPG was determined independently by three appraisers using the AGREE II (Appraisal of Guidelines for Research and Evaluation II) instrument.
Results
Twenty-two CPGs met the eligibility criteria. The median scores per AGREE II domain were: scope and purpose 90.7% (64.8–100%), stakeholder involvement 76.9% (27.8–96.3%); rigor of development 80.9% (27.1–92.4%); clarity of presentation 89.8% (50–100%); applicability 46.5% (12.5–87.5%); and editorial independence 91.7% (27.8–100%). Most of the CPGs (54.5%) were rated as “recommended with modifications” for clinical use.
Conclusions
Overall, the methodological quality of CPGs proving recommendations on the management of NSCLC is moderate, but there is still room for improvement in their development and implementation.

Similar content being viewed by others
References
Wild CP, Weiderpass E, Stewart BW. 2020 World Cancer Report: cancer research for cancer development: In Wild CP, Weiderpass E Stewart BW, (eds). International Agency for Research on Cancer. Lyon
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48:889–902.
Wu X, Denise B-B, Zhan F, Zhang J. Determining association between lung cancer mortality worldwide and risk factors using fuzzy inference modeling and random forest modeling. Int J Environ Res Public. 2022. https://doi.org/10.3390/ijerph192114161.
Cortés-Jofré M, Rueda JR, Asenjo-Lobos C, Madrid E, Bonfill CX. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2022. https://doi.org/10.1002/14651858.CD002141.pub3.
Cheng ES, Egger S, Hughes S, Weber M, Steinberg J, Rahman B, et al. Systematic review and meta-analysis of residential radon and lung cancer in never-smokers. Eur Respir Rev. 2021;30:1–14.
Relli V, Trerotola M, Guerra E, Alberti S. 2019 Abandoning the notion of non-small cell lung cancer. Trends Mol Med. 2019;25:585–94.
Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the of the TNM classification of lung cancer. J Thorac Oncol. 2017;12:1109–21.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73:17–48.
Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–40.
Hassan Murad M. Clinical practice guidelines a primer on development and dissemination. Mayo Clin Proc. 2017;92(423):33.
Hollon SD, Teachman BA. Advantages of developing clinical practice guidelines using international standards. Psychotherapy. 2019;56:340–6.
McAlister FA, Van Diepen S, Padwal RS, Johnson JA, Majumdar SR. How evidence-based are the recommendations in evidence-based guidelines? PLoS Med. 2007;4:1325–32.
Eikermann M, Holzmann N, Siering U, Rüther A. Tools for assessing the content of guidelines are needed to enable their effective use–a systematic comparison. BMC Res Notes. 2014;7(1):853.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010. https://doi.org/10.1503/cmaj.091716.
Alberta Health Services (AHS). Non-small cell lung cancer stage III. Clinical practice guideline LU-003. Alberta. 2012. www.albertahealthservices.ca
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cáncer american college of chest physicians evidence-based clinical practice guidelines. Chest. 2013. https://doi.org/10.1378/chest.12-2359.
Socinski MA, Evans T, Gettinger S, Hensing TA, Van Dam SL, Ireland B, et al. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. 2013. https://doi.org/10.1378/chest.12-2361.
Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cáncer american college of chest physicians evidence-based clinical practice guidelines. Chest. 2013. https://doi.org/10.1378/chest.12-2360.
Scottish Intercollegiate Guidelines Network (SIGN). Management of lung cancer a national clinical guideline. 2014. www.sign.ac.uk/pdf/sign50eqia.pdf.
Kulkarni S, Vella E, Coakley N, Cheng S, Gregg R, Ung YC, et al. Guideline the use of systemic treatment in the maintenance of patients with non-small cell lung cancer. J Thor Oncol. 2015. https://doi.org/10.1016/j.jtho.2016.03.007.
Álvarez FV, Trueba IM, Sanchis JB, López-Rodó LM, Rodríguez Suárez PM, de Cos Escuín JS, et al. Recommendations of the Spanish society of pneumology and thoracic Surgery on the diagnosis and treatment of non-small-cell lung cancer. Arch Bronconeumol. 2023;52(Suppl 1):2–62.
Ellis PM, Vella ET, Ung YC. Systemic treatment for patients with advanced non-small cell lung cáncer program in evidence-based care guideline version 3. Amsterdam: Elsevier; 2016.
Falkson CB, Vella ET, Yu E, El-Mallah M, Mackenzie R, Ellis PM, et al. Radiotherapy with curative intent in patients with early stage, medically inoperable, non-small cell lung cáncer Program in evidence-based care evidence-based Series. Current Oncol. 2017. https://doi.org/10.3747/co.24.3358.
Instituto Catalan de Oncología. ICOpraxis para el tratamiento médico de cáncer de pulmón de célula no pequeña. ICO. 2016;2:1–125.
Swaminath A, Vella ET, Ramchandar K, Robinson A, Simone C, Sun A, et al. Treatment of patients with stage III (N2 or N3) non-small cell lung cancer. guideline 7–3 version 3 a quality initiative of the program in evidence-based care (PEBC). Cancer Care Ontario. 2017. Available from: http://www.cancercare.on.ca/.
Moeller B, Balagamwala EH, Chen A, Creach KM, Giaccone G, Koshy M, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 update of an american society for radiation oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol Pract Radiat Oncol. 2018;8:245–50.
Majem M, Juan O, Insa A, Reguart N, Trigo JM, Carcereny E, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer. Clin Transl Oncol. 2018;21:3–17.
Facchinetti F, Pilotto S, Metro G, Baldini E, Bertolaccini L, Cappuzzo F, et al. Treatment of metastatic non-small cell lung cancer: 2018 guidelines of the Italian association of medical oncology (AIOM). Tumori. 2016;105:3–14.
Barrón-Barrón F, Guzmán-De Alba E, Alatorre-Alexander J, Aldaco-Sarvide F, Bautista-Aragón Y, Blake-Cerda M, et al. Guía de Práctica Clínica Nacional para el manejo del Cáncer de Pulmón de células no pequeñas en estadios tempranos, localmente avanzados y metastásicos. Salud Publica Mex. 2019;61:359.
Akamatsu H, Ninomiya K, Kenmotsu H, Morise M, Daga H, Goto Y, et al. The japanese lung cancer society guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–70.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up the ESMO guidelines committee. Ann Oncol. 2020;3(1):71.
Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol Ann Oncol. 2021;32:1637–42.
Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol. 2022;40:1356–84.
Pisters K, Kris MG, Gaspar LE, Ismaila N. Adjuvant systemic therapy and adjuvant radiation therapy for stage I-IIIA completely resected non-small-cell lung cancer: asco guideline rapid recommendation update. J Clin Oncol. 2022;40:1127–9.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. Natl Compr Canc Netw. 2022;20:497–530.
Singh N, Temin S, Baker S, Blanchard E, Brahmer JR, Celano P, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO living guideline. J Clin Oncol. 2022;40:3323–43.
Madera M, Franco J, Solà I, Bonfill X, Alonso-Coello P. Screening and diagnosis of oral cancer: a critical quality appraisal of clinical guidelines. Clin Oral Investig. 2019;23:2215–26.
Madera Anaya MV, Franco JV, Merchán-Galvis ÁM, Gallardo CR, Bonfill CX. Quality assessment of clinical practice guidelines on treatments for oral cancer. Cancer Treat Rev. 2018;65:47–53.
Santero M, Meade AG, Acosta-Dighero R, González L, Melendi S, Solà I, et al. European clinical practice guidelines on the use of chemotherapy for advanced oesophageal and gastric cancers: a critical review using the AGREE II and the AGREE-REX instruments. Clin Transl Oncol. 2022;24:1588–604.
Zhou X, Yang Y, Li C, Gu S, Hou W, Lai X, et al. What information can we gain from the quality appraisal of guidelines with physical activity recommendations for cancer patients A systematic review using the AGREE II and AGREE-REX tools. Supportive Care Cancer. 2023. https://doi.org/10.1007/s00520-022-07567-5.
Noyahr JK, Tatucu-Babet OA, Chapple LAS, Barlow CJ, Chapman MJ, Deane AM, et al. Methodological rigor and transparency in clinical practice guidelines for nutrition care in critically ill adults: a systematic review using the AGREE II and AGREE-REX tools. Nutrients. 2022. https://doi.org/10.3390/nu14132603.
Hoydonckx Y, Kumar P, Flamer D, Costanzi M, Raja SN, Peng P, et al. Quality of chronic pain interventional treatment guidelines from pain societies: assessment with the AGREE II instrument. Eur J Pain. 2020;24:704–21.
Al Wattar BH, Fisher M, Bevington L, Talaulikar V, Davies M, Conway G, et al. Clinical practice guidelines on the diagnosis and management of polycystic ovary syndrome: a systematic review and quality assessment study. J Clin Endocrinol Metab. 2021;6:2436–46.
Rabassa M, Hernández Ponce Y, Garcia-Ribera S, Johnston BC, Salvador Castell G, Manera M, et al. Food-based dietary guidelines in Spain: an assessment of their methodological quality. Eur J Clin Nutr. 2022;76:350–9.
Angel G, Trujillo C, Mallama M, Alonso-Coello P, Klimek M, Calvache JA. Methodological transparency of preoperative clinical practice guidelines for elective surgery. PLoS ONE. 2023. https://doi.org/10.1371/journal.pone.0272756.
Merchan-Galvis AM, Caicedo JP, Valencia-Payán CJ, Calvache JA. Methodological quality and transparency of clinical practice guidelines for difficult airway management using the appraisal of guidelines research & evaluation II instrument: a systematic review. Eur J Anaesthesiol. 2020;37:451–6.
Deana NF, Zaror C, Seiffert A, Aravena-Rivas Y, Muñoz-Millán P, Espinoza-Espinoza G, et al. Quality appraisal of clinical practice guidelines on provision of dental services during the first months of the COVID-19 pandemic. J Evid Based Dent Pract. 2021. https://doi.org/10.1016/j.jebdp.2021.101633.
Ng JY, Nault H, Nazir Z. Complementary and integrative medicine mention and recommendations: A systematic review and quality assessment of lung cancer clinical practice guidelines. Integr Med Res. 2021. https://doi.org/10.1016/j.imr.2020.100452.
Yang Y, Lu J, Ma Y, Xi C, Kang J, Zhang Q, et al. Evaluation of the reporting quality of clinical practice guidelines on lung cancer using the RIGHT checklist. Transl Lung Cancer Res. 2021;10:2588–602.
Yao X, Ma J, Wang Q, Kanters D, Ali MU, Florez ID. A comparison of agree and right: which clinical practice guideline reporting checklist should be followed by guideline developers? J Gen Intern Med. 2020;35:894–8.
Chen YP, Wang YQ, Li WF, Chen L, Xu C, Lu TX, et al. Critical evaluation of the quality and recommendations of clinical practice guidelines for nasopharyngeal carcinoma. J Natl Compr Canc. 2017;15:336–44.
Seiffert A, Zaror C, Atala-Acevedo C, Ormeño A, Martínez-Zapata MJ, Alonso-Coello P. Dental caries prevention in children and adolescents: a systematic quality assessment of clinical practice guidelines. Clin Oral Investig. 2018;22:3129–41.
Jolliffe L, Lannin NA, Cadilhac DA, Hoffmann T. Systematic review of clinical practice guidelines to identify recommendations for rehabilitation after stroke and other acquired brain injuries. BMJ Open. 2018. https://doi.org/10.1136/bmjopen-2017-018791.
Yadav P, Alsabban A, de Los RT, Varghese A, Ming JM, Milford K, et al. A systematic review of paediatric neurogenic lower urinary tract dysfunction guidelines using the appraisal of guidelines and research evaluation (AGREE) II instrument. BJU. 2018;13(5):520.
Simancas-Racines D, Montero-Oleas N, Vernooij RWM, Arevalo-Rodriguez I, Fuentes P, Gich I, et al. Quality of clinical practice guidelines about red blood cell transfusion. J Evid Based Med. J Evid Based Med. 2019;12:113–24.
Alarcon JD, Rubiano AM, Chirinos MS, Valderrama A, Gich I, Bonfill X, et al. Clinical practice guidelines for the care of patients with severe traumatic brain injury: a systematic evaluation of their quality. J Trauma Acute Care Surg. 2013;75:311–9.
Alonso-Coello P, Irfan A, Solà I, Gich I, Delgado-Noguera M, Rigau D, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2022. https://doi.org/10.1136/qshc.2010.042077.
Kwah LK, Green J, Butler J, Lam L. Quality of clinical practice guidelines for management of limb amputations: a systematic review. Phys Ther. 2019;99:577–90.
Fuentes Padilla P, Martínez G, Vernooij RWM, Cosp XB, Alonso-Coello P. Nutrition in critically ill adults: a systematic quality assessment of clinical practice guidelines. Clin Nutr. 2016;35:1219–25.
Funding
There was no source of funding involved in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cortés-Jofré, M., Madera, M., Tirado-Amador, L. et al. Treatments for non-small cell lung cancer: a systematic quality assessment of clinical practice guidelines. Clin Transl Oncol 25, 3541–3555 (2023). https://doi.org/10.1007/s12094-023-03223-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03223-4