Abstract
Introduction
In the present study, we sought to clarify the role of LINC01119 delivered by cancer-associated adipocytes (CAAs)-derived exosomes (CAA-Exo) and its mechanistic actions in ovarian cancer (OC).
Materials and methods
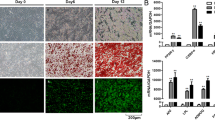
The expression of LINC01119 was determined in OC, and the relationship between LINC01119 expression and the prognosis of OC patients was analyzed. Besides, 3D co-culture cell models were constructed using green fluorescent protein-labeled OC cells and red fluorescent protein-labeled mature adipocytes. Mature adipocytes were co-cultured with OC cells to induce CAA. Macrophages treated with CAA-Exo were co-cultured with SKOV3 cells following ectopic expression and depletion experiments of LINC01119 and SOCS5 to detect M2 polarization of macrophages, PD-L1 level, proliferation of CD3+ T cells, and cytotoxicity of T cells to SKOV3 cells.
Results
LINC01119 was elevated in the plasma Exo of OC patients, which was related to shorter overall survival in OC patients. LINC01119 expression was increased in CAA-Exo, which could upregulate SOCS5 in OC. Finally, CAA-Exo carrying LINC01119 induced M2 polarization of macrophages to promote immune escape in OC, as evidenced by inhibited CD3+ T cell proliferation, increased PD-L1 level, and attenuated T cell toxicity to SKOV3 cells.
Conclusion
In conclusion, the key findings of the current study demonstrated the promoting effects of CAA-Exo containing LINC01119 mediating SOCS5 on M2 polarization of macrophages and immune escape in OC.







Similar content being viewed by others
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371: m3773.
Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, Jin WL. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Front Immunol. 2020;11: 577869.
Gao Y, Xu Y, Zhao S, Qian L, Song T, Zheng J, et al. Growth differentiation factor-15 promotes immune escape of ovarian cancer via targeting CD44 in dendritic cells. Exp Cell Res. 2021;402: 112522.
Qian M, Ling W, Ruan Z. Long non-coding RNA SNHG12 promotes immune escape of ovarian cancer cells through their crosstalk with M2 macrophages. Aging (Albany NY). 2020;12:17122–36.
Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells. 2020;9.
Song M, Yeku OO, Rafiq S, Purdon T, Dong X, Zhu L, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 2020;11:6298.
Yao H, He S. Multi‑faceted role of cancer‑associated adipocytes in the tumor microenvironment (review). Mol Med Rep. 2021;24.
Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12:95.
Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38:81.
Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding C, et al. The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 cells. Biomed Res Int. 2019;2019:2595801.
Zhang Y, Wei YJ, Zhang YF, Liu HW, Zhang YF. Emerging functions and clinical applications of exosomal ncRNAs in ovarian cancer. Front Oncol. 2021;11: 765458.
Liang H, Yu T, Han Y, Jiang H, Wang C, You T, et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer. 2018;17:119.
Wang JY, Lu AQ, Chen LJ. LncRNAs in ovarian cancer. Clin Chim Acta. 2019;490:17–27.
Tu Z, Schmoellerl J, Mariani O, Zheng Y, Hu Y, Vincent-Salomon A, et al. The LINC01119-SOCS5 axis as a critical theranostic in triple-negative breast cancer. NPJ Breast Cancer. 2021;7:69.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2014;5:614.
Yang M, Chen H, Zhou L, Huang X, Su F, Wang P. Identification of SOCS family members with prognostic values in human ovarian cancer. Am J Transl Res. 2020;12:1824–38.
Takehara M, Sato Y, Kimura T, Noda K, Miyamoto H, Fujino Y, et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Sci. 2020;111:2883–94.
Wang Z, He J, Bach DH, Huang YH, Li Z, Liu H, et al. Induction of m(6)A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. J Exp Clin Cancer Res. 2022;41:4.
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and Beiging in white adipose tissue. Diabetes. 2018;67:235–47.
Wu Q, Li B, Li J, Sun S, Yuan J, Sun S. Cancer-associated adipocytes as immunomodulators in cancer. Biomark Res. 2021;9:2.
He L, Jhong JH, Chen Q, Huang KY, Strittmatter K, Kreuzer J, et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37: 109955.
Bogel G, Muranyi J, Szokol B, Kukor Z, Mora I, Kardon T, et al. Production of NOS2 and inflammatory cytokines is reduced by selected protein kinase inhibitors with partial repolarization of HL-60 derived and human blood macrophages. Heliyon. 2022;8: e08670.
Mohapatra S, Pioppini C, Ozpolat B, Calin GA. Non-coding RNAs regulation of macrophage polarization in cancer. Mol Cancer. 2021;20:24.
Zhao X, Sun L, Mu T, Yi J, Ma C, Xie H, et al. An HBV-encoded miRNA activates innate immunity to restrict HBV replication. J Mol Cell Biol. 2020;12:263–76.
Zou X, Zhao Y, Liang X, Wang H, Zhu Y, Shao Q. Double insurance for OC: miRNA-mediated platinum resistance and immune escape. Front Immunol. 2021;12: 641937.
John B, Naczki C, Patel C, Ghoneum A, Qasem S, Salih Z, et al. Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC. Oncogene. 2019;38:4366–83.
Pascual-Anton L, Cardenes B, Sainz de la Cuesta R, Gonzalez-Cortijo L, Lopez-Cabrera M, Cabanas C, et al. Mesothelial-to-mesenchymal transition and exosomes in peritoneal metastasis of ovarian cancer. Int J Mol Sci. 2021;22.
Tian W, Lei N, Zhou J, Chen M, Guo R, Qin B, et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022;13:64.
Shang A, Wang W, Gu C, Chen C, Zeng B, Yang Y, et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J Exp Clin Cancer Res. 2019;38:411.
Acknowledgements
Not applicable.
Funding
This study was supported by Medical Key Supporting Project of Suzhou City (no. SZFCXK202142); Key Projects of Medical and Health Science and Technology Plan of Suzhou Hi-tech Zone (2020Z008) and Suzhou Science and Technology Development Plan (no. SKJYD2021058).
Author information
Authors and Affiliations
Contributions
QLZ, JZ and DHG designed the study. YXL, WJD, XD and XLD collated the data, carried out data analyses and produced the initial draft of the manuscript. QLZ, JZ and DHG contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This study was approved by the Ethics Committee of Suzhou Science & Technology Town Hospital and the methods were carried out in accordance with the approved guidelines. All the patients have been informed and signed informed consent before the experiments.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2023_3185_MOESM1_ESM.tif
Figure S1 Effect of M2 macrophage polarization inhibitors on immune escape of OC cells. A, PD-L1 protein level in SKOV3 cells measured by Western blot analysis. B, The cytotoxicity of T cells to SKOV3 cells detected by LDH release assay. C, CFSE-labeled T cell proliferation assessed by flow cytometry. ns p>0.05; **p<0.05; **p<0.01; ***p<0.001. All cell experiments were repeated three times (TIFF 20,761 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Q., Zhang, J., Liu, Y. et al. LINC01119 encapsulated by cancer-associated adipocytes-derived exosomes promotes M2 polarization of macrophages to induce immune escape in ovarian cancer in a 3D co-culture cell-based model. Clin Transl Oncol 25, 3174–3187 (2023). https://doi.org/10.1007/s12094-023-03185-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03185-7