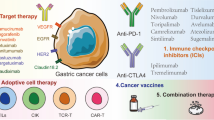
Abstract
Although the incidence rate and mortality of gastric/gastroesophageal cancer (G/GEJC) are declining globally, G/GEJC remains a health issue in East Asia. When diagnosed as advanced stage, treatment after serial lines of chemotherapy is limited, with a median overall survival of less than 1 year. Immunotherapy, including immune checkpoint inhibitors (ICIs) and cellular immunotherapy, has changed the prospects of cancer therapy by reversing immune suppression in the tumor microenvironment. As part of this review, we enumerated the clinical uses of ICIs related to the immunosuppressive signaling axis PD-1/PD-L1 and CTLA-4/B7. ICIs were initially approved as a secondary treatment option for patients with severe pretreating advanced gastric and gastroesophageal cancer (AG/GEJC). Till now, it has become the mainstream therapy in combination with chemotherapy and targeted therapy for patients identified by biomarkers. Numerous evidence showed microsatellite instability (MSI), programmed cell death ligand 1 (PD-L1) expression, tumor mutation burden (TMB) and Epstein–Barr virus (EBV) status might be indicative to the use of ICIs. In addition, we discussed the current limitations and prospects of ICIs in AG/GGEJC, as well as the first clinical application of novel CAR-T cell therapies.

Similar content being viewed by others
Data availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Wang F, Zhang X, Li Y, Tang L, Qu X, Ying J, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41:747–95. https://doi.org/10.1002/cac2.12193.
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212–39. https://doi.org/10.1038/ajg.2016.563.
Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. https://doi.org/10.1056/NEJMra020542.
Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, et al. Effect of helicobacter pylori eradication on gastric cancer prevention: updated report from a randomized controlled trial with 26.5 years of follow-up. Gastroenterology. 2022;163:154-162.e3. https://doi.org/10.1053/j.gastro.2022.03.039.
Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–8. https://doi.org/10.1055/s-2005-861352.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. https://doi.org/10.1038/nature13480.
Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264–79. https://doi.org/10.3322/caac.21657.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. https://doi.org/10.1056/NEJMoa073149.
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:4438–44. https://doi.org/10.1200/JCO.2012.48.5805.
Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau H-T, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–48. https://doi.org/10.1016/S1470-2045(18)30739-3.
Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai B-C, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. https://doi.org/10.1002/14651858.CD004064.pub4.
Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet Lond Engl. 2010;376:687–97. https://doi.org/10.1016/S0140-6736(10)61121-X.
Vakiani E. HER2 testing in gastric and gastroesophageal adenocarcinomas. Adv Anat Pathol. 2015;22:194–201. https://doi.org/10.1097/PAP.0000000000000067.
Seo S, Ryu M-H, Park YS, Ahn JY, Park Y, Park SR, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2019;22:527–35. https://doi.org/10.1007/s10120-018-0891-1.
Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–30. https://doi.org/10.1056/NEJMoa2004413.
Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188549. https://doi.org/10.1016/j.bbcan.2021.188549.
Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. https://doi.org/10.1016/S1470-2045(14)70420-6.
Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0182692. https://doi.org/10.1371/journal.pone.0182692.
Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2020;23:95–104. https://doi.org/10.1007/s10120-019-00999-9.
K K, Z N, C E, G L, W S, J D, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019. https://doi.org/10.5858/arpa.2018-0043-OA.
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26. https://doi.org/10.1016/S1470-2045(16)00175-3.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. https://doi.org/10.1001/jamaoncol.2018.0013.
Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet Lond Engl. 2018;392:123–33. https://doi.org/10.1016/S0140-6736(18)31257-1.
Shitara K, Van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–80. https://doi.org/10.1001/jamaoncol.2020.3370.
Chung HC, Kang Y-K, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, et al. Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): a randomized, open-label, phase 3 trial in Asian patients. Cancer. 2022;128:995–1003. https://doi.org/10.1002/cncr.34019.
Wainberg ZA, Fuchs CS, Tabernero J, Shitara K, Muro K, Van Cutsem E, et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score ≥10. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27:1923–31. https://doi.org/10.1158/1078-0432.CCR-20-2980.
Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7:895. https://doi.org/10.1001/jamaoncol.2021.0275.
Fuchs CS, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandala M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2022;25:197–206. https://doi.org/10.1007/s10120-021-01227-z.
Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesophageal adenocarcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:1127–36. https://doi.org/10.1016/j.annonc.2021.05.803.
Topp BG, Thiagarajan K, De Alwis DP, Snyder A, Hellmann MD. Lesion-level heterogeneity of radiologic progression in patients treated with pembrolizumab. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:1618–25. https://doi.org/10.1016/j.annonc.2021.09.006.
Bang Y-J, Kang Y-K, Catenacci DV, Muro K, Fuchs CS, Geva R, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2019;22:828–37. https://doi.org/10.1007/s10120-018-00909-5.
Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, et al. (2020) Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer Oxf Engl. 1990;129:97–106. https://doi.org/10.1016/j.ejca.2020.02.002.
Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–31. https://doi.org/10.1016/S1470-2045(20)30169-8.
Lee C-K, Rha SY, Kim HS, Jung M, Kang B, Che J, et al. A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer. Nat Commun. 2022;13:6002. https://doi.org/10.1038/s41467-022-33267-z.
Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–30. https://doi.org/10.1038/s41586-021-04161-3.
Catenacci DVT, Kang Y-K, Park H, Uronis HE, Lee K-W, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21:1066–76. https://doi.org/10.1016/S1470-2045(20)30326-0.
Herbst RS, Arkenau H-T, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20:1109–23. https://doi.org/10.1016/S1470-2045(19)30458-9.
Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057–65. https://doi.org/10.1016/S1470-2045(20)30271-0.
Varadan V, Gilmore H, Miskimen KLS, Tuck D, Parsai S, Awadallah A, et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperativetrastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:3249–59. https://doi.org/10.1158/1078-0432.CCR-15-2021.
Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2017;390:2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5.
Kang Y-K, Chen L-T, Ryu M-H, Oh D-Y, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47. https://doi.org/10.1016/S1470-2045(21)00692-6.
Boku N, Ryu M-H, Kato K, Chung HC, Minashi K, Lee K-W, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol Off J Eur Soc Med Oncol. 2019;30:250–8. https://doi.org/10.1093/annonc/mdy540.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet Lond Engl. 2021;398:27–40. https://doi.org/10.1016/S0140-6736(21)00797-2.
Chon HJ, Hyung WJ, Kim C, Park S, Kim J-H, Park CH, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg. 2017;265:946–53. https://doi.org/10.1097/SLA.0000000000001793.
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol Off J Am Soc Clin Oncol. 2020;38:2053–61. https://doi.org/10.1200/JCO.19.03296.
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. https://doi.org/10.1136/bmj-2021-068714.
Salem ME, Puccini A, Xiu J, Raghavan D, Lenz H-J, Korn WM, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23:1319–27. https://doi.org/10.1634/theoncologist.2018-0143.
Jiang H, Zheng Y, Qian J, Mao C, Xu X, Li N, et al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial. BMC Cancer. 2020;20:760. https://doi.org/10.1186/s12885-020-07251-z.
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32:S1331. https://doi.org/10.1016/j.annonc.2021.08.2133.
Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538–45. https://doi.org/10.1038/s41416-018-0100-3.
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:515–23. https://doi.org/10.1158/1078-0432.CCR-18-2484.
Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27:3069–78. https://doi.org/10.1158/1078-0432.CCR-20-4691.
Jing C, Wang J, Zhu M, Bai Z, Zhao B, Zhang J, et al. Camrelizumab combined with apatinib and S-1 as second-line treatment for patients with advanced gastric or gastroesophageal junction adenocarcinoma: a phase 2, single-arm, prospective study. Cancer Immunol Immunother CII. 2022;71:2597–608. https://doi.org/10.1007/s00262-022-03174-9.
Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8:e000437. https://doi.org/10.1136/jitc-2019-000437.
Desai J, Deva S, Lee JS, Lin C-C, Yen C-J, Chao Y, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8:e000453. https://doi.org/10.1136/jitc-2019-000453.
Xu J, Bai Y, Xu N, Li E, Wang B, Wang J, et al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26:4542–50. https://doi.org/10.1158/1078-0432.CCR-19-3561.
Lu Z, Yang S, Luo X, Shi Y, Lee J-S, Deva S, et al. The combination of gene hyperamplification and PD-L1 expression as a biomarker for the clinical benefit of tislelizumab in gastric/gastroesophageal junction adenocarcinoma. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2022;25:943–55. https://doi.org/10.1007/s10120-022-01308-7.
Zheng Y, Mislang ARA, Coward J, Cosman R, Cooper A, Underhill C, et al. Penpulimab, an anti-PD1 IgG1 antibody in the treatment of advanced or metastatic upper gastrointestinal cancers. Cancer Immunol Immunother CII. 2022;71:2371–9. https://doi.org/10.1007/s00262-022-03160-1.
Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:1479–86. https://doi.org/10.1093/annonc/mdz197.
Jiang M, Zhang C, Hu Y, Li T, Yang G, Wang G, et al. Anlotinib combined with toripalimab as second-line therapy for advanced, relapsed gastric or gastroesophageal junction carcinoma. Oncologist. 2022;27:e856–69. https://doi.org/10.1093/oncolo/oyac136.
Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. https://doi.org/10.1056/NEJMoa1200694.
Bang Y-J, Ruiz EY, Van Cutsem E, Lee K-W, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:2052–60. https://doi.org/10.1093/annonc/mdy264.
Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, et al. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2019;22:817–27. https://doi.org/10.1007/s10120-018-0903-1.
Chung HC, Arkenau H-T, Lee J, Rha SY, Oh D-Y, Wyrwicz L, et al. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2019;7:30. https://doi.org/10.1186/s40425-019-0508-1.
Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu M-H, Muntean AS, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric Cancers: results from JAVELIN gastric 100. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39:966–77. https://doi.org/10.1200/JCO.20.00892.
Kelly RJ, Lee J, Bang Y-J, Almhanna K, Blum-Murphy M, Catenacci DVT, et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26:846–54. https://doi.org/10.1158/1078-0432.CCR-19-2443.
Bang Y-J, Golan T, Dahan L, Fu S, Moreno V, Park K, et al. 2020) Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: an open-label, phase Ia/b study (JVDJ. Eur J Cancer Oxf Engl. 1990;137:272–84. https://doi.org/10.1016/j.ejca.2020.06.007.
Evrard C, Aparicio T, Soularue E, Le Malicot K, Desramé J, Botsen D, et al. Safety of FOLFIRI + durvalumab +/- tremelimumab in second line of patients with advanced gastric cancer: a safety run-in from the randomized phase II study DURIGAST PRODIGE 59. Biomedicines. 2022;10:1211. https://doi.org/10.3390/biomedicines10051211.
Kwon M, Kim G, Kim R, Kim K-T, Kim ST, Smith S, et al. Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced gastric cancer. J Immunother Cancer. 2022;10:e005041. https://doi.org/10.1136/jitc-2022-005041.
Li J, Deng Y, Zhang W, Zhou A-P, Guo W, Yang J, et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J Hematol OncolJ Hematol Oncol. 2021;14:95. https://doi.org/10.1186/s13045-021-01095-1.
Gong J, Cao J, Zhang Q, Xu N, Zhao Y, Xing B, et al. Safety, antitumor activity and biomarkers of sugemalimab in Chinese patients with advanced solid tumors or lymphomas: results from the first-in-human phase 1 trial. Cancer Immunol Immunother CII. 2022;71:1897–908. https://doi.org/10.1007/s00262-021-03102-3.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. https://doi.org/10.1056/NEJMoa1104621.
Bang Y-J, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:5671–8. https://doi.org/10.1158/1078-0432.CCR-17-0025.
Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36:2836–44. https://doi.org/10.1200/JCO.2017.76.6212.
Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942–8. https://doi.org/10.1038/s41586-022-04508-4.
Ralph C, Elkord E, Burt DJ, O’Dwyer JF, Austin EB, Stern PL, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16:1662–72. https://doi.org/10.1158/1078-0432.CCR-09-2870.
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15:2548–60. https://doi.org/10.7150/ijbs.34213.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99. https://doi.org/10.1038/s41573-019-0051-2.
Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:7624–34. https://doi.org/10.1158/1078-0432.CCR-08-1547.
Dottermusch M, Krüger S, Behrens H-M, Halske C, Röcken C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch Int J Pathol. 2019;475:563–71. https://doi.org/10.1007/s00428-019-02624-7.
Türeci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:1487–95. https://doi.org/10.1093/annonc/mdz199.
Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:609–19. https://doi.org/10.1016/j.annonc.2021.02.005.
Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111:409–18. https://doi.org/10.1093/jnci/djy134.
Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28:1189–98. https://doi.org/10.1038/s41591-022-01800-8.
Bębnowska D, Grywalska E, Niedźwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, et al. CAR-T cell therapy-an overview of targets in gastric cancer. J Clin Med. 2020;9:1894. https://doi.org/10.3390/jcm9061894.
S ML, S M, W L, R CA, J K, M-T C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol Off J Eur Soc Med Oncol. 2021. https://doi.org/10.1016/j.annonc.2020.10.478.
M F, S L, S GP, L F, M M, F M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019. https://doi.org/10.1038/s41571-019-0218-0.
Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40:392–402. https://doi.org/10.1200/JCO.21.01862.
M A, F M, L J, S M, S-F R, N K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020. https://doi.org/10.1016/S1470-2045(20)30445-9.
Addeo A, Friedlaender A, Banna GL, Weiss GJ. TMB or not TMB as a biomarker: that is the question. Crit Rev Oncol Hematol. 2021;163:103374. https://doi.org/10.1016/j.critrevonc.2021.103374.
Hagi T, Kurokawa Y, Kawabata R, Omori T, Matsuyama J, Fujitani K, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer. 2020;123:965–72. https://doi.org/10.1038/s41416-020-0975-7.
Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer - PubMed. https://pubmed.ncbi.nlm.nih.gov/30028179/. Accessed 11 Jan 2023
Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen H-Z, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017. https://doi.org/10.1200/PO.17.00073.
Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45–62. https://doi.org/10.1016/j.pharmthera.2018.04.004.
Kok M, Chalabi M, Haanen J. How I treat MSI cancers with advanced disease. ESMO Open. 2019;4:e000511. https://doi.org/10.1136/esmoopen-2019-000511.
McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:661–72. https://doi.org/10.1016/j.annonc.2021.02.006.
Bai Y, Xie T, Wang Z, Tong S, Zhao X, Zhao F, et al. Efficacy and predictive biomarkers of immunotherapy in Epstein-Barr virus-associated gastric cancer. J Immunother Cancer. 2022;10:e004080. https://doi.org/10.1136/jitc-2021-004080.
Funding
This article received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, all authors; writing—original draft preparation, RC; writing—review and editing, RC, BL, HW, YZ. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, R., Li, B., Wang, H. et al. Immune checkpoint inhibitors and cellular immunotherapy for advanced gastric, gastroesophageal cancer: a long pathway. Clin Transl Oncol 25, 3122–3138 (2023). https://doi.org/10.1007/s12094-023-03181-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03181-x