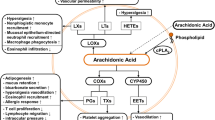
Abstract
Radiotherapy is one of the main therapies for cancer. The process leading to radioresistance is still not fully understood. Cancer radiosensitivity is related to the DNA reparation of cancer cells and the tumor microenvironment (TME), which supports cancer cell survival. Factors that affect DNA reparation and the TME can directly or indirectly affect the radiosensitivity of cancer. Recent studies have shown that lipid metabolism in cancer cells, which is involved in the stability of cell membrane structure, energy supply and signal transduction of cancer cells, can also affect the phenotype and function of immune cells and stromal cells in the TME. In this review, we discussed the effects of lipid metabolism on the radiobiological characteristics of cancer cells and the TME. We also summarized recent advances in targeted lipid metabolism as a radiosensitizer and discussed how these scientific findings could be translated into clinical practice to improve the radiosensitivity of cancer.


Similar content being viewed by others
Data availability
Not Applicable.
References
Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9:688–99. https://doi.org/10.1038/nrclinonc.2012.194.
Kim W, Lee S, Seo D, Kim D, Kim K, Kim E, et al. Cellular stress responses in radiotherapy. Cells. 2019. https://doi.org/10.3390/cells8091105.
Morgan MA, Lawrence TS. Molecular pathways: overcoming radiation resistance by targeting DNA damage response pathways. Clin Cancer Res. 2015;21:2898–904. https://doi.org/10.1158/1078-0432.CCR-13-3229.
Darragh LB, Oweida AJ, Karam SD. Overcoming resistance to combination radiation-immunotherapy: a focus on contributing pathways within the tumor microenvironment. Front Immunol. 2018;9:3154. https://doi.org/10.3389/fimmu.2018.03154.
Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–35.
Chen D, Menon H, Verma V, Guo C, Ramapriyan R, Barsoumian H, et al. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2019-000492.
Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611–8. https://doi.org/10.1200/JCO.2017.76.2229.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. https://doi.org/10.1056/NEJMoa1809697.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–9. https://doi.org/10.1038/ncb3124.
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28. https://doi.org/10.1186/s12943-021-01316-8.
Martinez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–80. https://doi.org/10.1038/s41568-021-00378-6.
Piper M, Mueller AC, Karam SD. The interplay between cancer associated fibroblasts and immune cells in the context of radiation therapy. Mol Carcinog. 2020;59:754–65. https://doi.org/10.1002/mc.23205.
Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 2020;31:62–76. https://doi.org/10.1016/j.cmet.2019.11.010.
Currie E, Schulze A, Zechner R, Walther TC, Farese RV. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–61. https://doi.org/10.1016/j.cmet.2013.05.017.
Grabner GF, Xie H, Schweiger M, Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab. 2021;3:1445–65. https://doi.org/10.1038/s42255-021-00493-6.
Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, et al. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122–31. https://doi.org/10.1074/jbc.M702854200.
Madak-Erdogan Z, Band S, Zhao YC, Smith BP, Kulkoyluoglu-Cotul E, Zuo Q, et al. Free fatty acids rewire cancer metabolism in obesity-associated breast cancer via estrogen receptor and mTOR signaling. Cancer Res. 2019;79:2494–510. https://doi.org/10.1158/0008-5472.CAN-18-2849.
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–83. https://doi.org/10.1053/j.gastro.2010.12.006.
Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–25. https://doi.org/10.1158/0008-5472.CAN-05-0571.
Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–94. https://doi.org/10.1158/0008-5472.CAN-05-1489.
De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–804.
Mashima T, Oh-hara T, Sato S, Mochizuki M, Sugimoto Y, Yamazaki K, et al. p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target. J Natl Cancer Inst. 2005;97:765–77. https://doi.org/10.1093/jnci/dji133.
Wang J, Li Y. CD36 tango in cancer: signaling pathways and functions. Theranostics. 2019;9:4893–908. https://doi.org/10.7150/thno.36037.
Watt MJ, Clark AK, Selth LA, Haynes VR, Lister N, Rebello R, et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aau5758.
Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–5. https://doi.org/10.1038/nature20791.
Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. https://doi.org/10.1038/srep14752.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. https://doi.org/10.1038/nm.2492.
Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–69. https://doi.org/10.1158/2159-8290.CD-15-1177.
Wen YA, Xing X, Harris JW, Zaytseva YY, Mitov MI, Napier DL, et al. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. https://doi.org/10.1038/cddis.2017.21.
Panaroni C, Fulzele K, Mori T, Siu KT, Onyewadume C, Maebius A, et al. Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood. 2022;139:876–88. https://doi.org/10.1182/blood.2021013832.
Tucci J, Chen T, Margulis K, Orgel E, Paszkiewicz RL, Cohen MD, et al. Adipocytes provide fatty acids to acute lymphoblastic leukemia cells. Front Oncol. 2021;11:665763. https://doi.org/10.3389/fonc.2021.665763.
Pham DV, Park PH. Adiponectin triggers breast cancer cell death via fatty acid metabolic reprogramming. J Exp Clin Cancer Res. 2022;41:9. https://doi.org/10.1186/s13046-021-02223-y.
Cheng X, Geng F, Pan M, Wu X, Zhong Y, Wang C, et al. Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab. 2020;32:229-242 e8. https://doi.org/10.1016/j.cmet.2020.06.002.
Xu Y, Qian SY. Anti-cancer activities of omega-6 polyunsaturated fatty acids. Biomed J. 2014;37:112–9. https://doi.org/10.4103/2319-4170.131378.
Tanaka A, Yamamoto A, Murota K, Tsujiuchi T, Iwamori M, Fukushima N. Polyunsaturated fatty acids induce ovarian cancer cell death through ROS-dependent MAP kinase activation. Biochem Biophys Res Commun. 2017;493:468–73. https://doi.org/10.1016/j.bbrc.2017.08.168.
Liu Z, Hopkins MM, Zhang Z, Quisenberry CB, Fix LC, Galvan BM, et al. Omega-3 fatty acids and other FFA4 agonists inhibit growth factor signaling in human prostate cancer cells. J Pharmacol Exp Ther. 2015;352:380–94. https://doi.org/10.1124/jpet.114.218974.
Hopkins MM, Meier KE. Free fatty acid receptor (FFAR) agonists inhibit proliferation of human ovarian cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2017;122:24–9. https://doi.org/10.1016/j.plefa.2017.06.013.
Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–26. https://doi.org/10.1158/0008-5472.CAN-09-3871.
Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4:594–610. https://doi.org/10.1038/nrd1776.
Bandu R, Mok HJ, Kim KP. Phospholipids as cancer biomarkers: mass spectrometry-based analysis. Mass Spectrom Rev. 2018;37:107–38. https://doi.org/10.1002/mas.21510.
Shafiee MN, Ortori CA, Barrett DA, Mongan NP, Abu J, Atiomo W. Lipidomic biomarkers in polycystic ovary syndrome and endometrial cancer. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21134753.
Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, Martinez-Pineiro L, Sanchez JJ, Bonilla F, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296:580–3. https://doi.org/10.1016/s0006-291x(02)00920-8.
Mika A, Pakiet A, Czumaj A, Kaczynski Z, Liakh I, Kobiela J, et al. Decreased triacylglycerol content and elevated contents of cell membrane lipids in colorectal cancer tissue: a lipidomic study. J Clin Med. 2020. https://doi.org/10.3390/jcm9041095.
Chang W, Fa H, Xiao D, Wang J. Targeting phosphatidylserine for cancer therapy: prospects and challenges. Theranostics. 2020;10:9214–29. https://doi.org/10.7150/thno.45125.
Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18:33–50. https://doi.org/10.1038/nrc.2017.96.
Vaena S, Chakraborty P, Lee HG, Janneh AH, Kassir MF, Beeson G, et al. Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response. Cell Rep. 2021;35: 109076. https://doi.org/10.1016/j.celrep.2021.109076.
Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82(82):103–26. https://doi.org/10.1146/annurev-physiol-021119-034627.
Urbanelli L, Buratta S, Logozzi M, Mitro N, Sagini K, Di Raimo RD, et al. Lipidomic analysis of cancer cells cultivated at acidic pH reveals phospholipid fatty acids remodelling associated with transcriptional reprogramming. J Enzyme Inhib Med Chem. 2020;35:963–73. https://doi.org/10.1080/14756366.2020.1748025.
Li YJ, Fahrmann JF, Aftabizadeh M, Zhao Q, Tripathi SC, Zhang C, et al. Fatty acid oxidation protects cancer cells from apoptosis by increasing mitochondrial membrane lipids. Cell Rep. 2022;39: 111044. https://doi.org/10.1016/j.celrep.2022.111044.
Boyd NF, McGuire V. Evidence of association between plasma high-density lipoprotein cholesterol and risk factors for breast cancer. J Natl Cancer Inst. 1990;82:460–8. https://doi.org/10.1093/jnci/82.6.460.
Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021;14: 101043. https://doi.org/10.1016/j.tranon.2021.101043.
Wang XX, Xu WJ, Zhan P, Xu TX, Jin JJ, Miu YY, et al. Overexpression of geranylgeranyl diphosphate synthase contributes to tumour metastasis and correlates with poor prognosis of lung adenocarcinoma. J Cell Mol Med. 2018;22:2177–89. https://doi.org/10.1111/jcmm.13493.
Todenhofer T, Hennenlotter J, Kuhs U, Gerber V, Gakis G, Vogel U, et al. Altered expression of farnesyl pyrophosphate synthase in prostate cancer: evidence for a role of the mevalonate pathway in disease progression? World J Urol. 2013;31:345–50. https://doi.org/10.1007/s00345-012-0844-y.
Baek AE, Nelson ER. The contribution of cholesterol and its metabolites to the pathophysiology of breast cancer. Horm Cancer. 2016;7:219–28. https://doi.org/10.1007/s12672-016-0262-5.
Zhang GM, Chen W, Yao Y, Luo L, Sun LJ. LDLR promotes growth and invasion in renal cell carcinoma and activates the EGFR pathway. Neoplasma. 2022;69:113–22. https://doi.org/10.4149/neo_2021_210607N762.
Gallagher EJ, Zelenko Z, Neel BA, Antoniou IM, Rajan L, Kase N, et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36:6462–71. https://doi.org/10.1038/onc.2017.247.
de Gonzalo-Calvo D, Lopez-Vilaro L, Nasarre L, Perez-Olabarria M, Vazquez T, Escuin D, et al. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. 2015;15:460. https://doi.org/10.1186/s12885-015-1469-5.
Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:580–90. https://doi.org/10.1016/j.celrep.2012.08.011.
Bovenga F, Sabba C, Moschetta A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015;21:517–26. https://doi.org/10.1016/j.cmet.2015.03.002.
Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol. 2018;14:452–63. https://doi.org/10.1038/s41574-018-0037-x.
Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case–control study. Cancer. 2004;100:2308–16. https://doi.org/10.1002/cncr.20271.
Nayan M, Punjani N, Juurlink DN, Finelli A, Austin PC, Kulkarni GS, et al. Statin use and kidney cancer survival outcomes: a systematic review and meta-analysis. Cancer Treat Rev. 2017;52:105–16. https://doi.org/10.1016/j.ctrv.2016.11.009.
Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The current evidence on statin use and prostate cancer prevention: are we there yet? Nat Rev Urol. 2017;14:107–19. https://doi.org/10.1038/nrurol.2016.199.
Wu Y, Si R, Tang H, He Z, Zhu H, Wang L, et al. Cholesterol reduces the sensitivity to platinum-based chemotherapy via upregulating ABCG2 in lung adenocarcinoma. Biochem Biophys Res Commun. 2015;457:614–20. https://doi.org/10.1016/j.bbrc.2015.01.035.
Mok EHK, Leung CON, Zhou L, Lei MML, Leung HW, Tong M, et al. Caspase-3-induced activation of SREBP2 drives drug resistance via promotion of cholesterol biosynthesis in hepatocellular carcinoma. Cancer Res. 2022;82:3102–15. https://doi.org/10.1158/0008-5472.CAN-21-2934.
Wang X, Sun B, Wei L, Jian X, Shan K, He Q, et al. Cholesterol and saturated fatty acids synergistically promote the malignant progression of prostate cancer. Neoplasia. 2022;24:86–97. https://doi.org/10.1016/j.neo.2021.11.004.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18. https://doi.org/10.1038/cr.2016.151.
Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–65. https://doi.org/10.1016/j.it.2016.09.006.
Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–62. https://doi.org/10.1016/j.ccell.2018.03.012.
Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. https://doi.org/10.1038/ncomms7692.
Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306–11. https://doi.org/10.1038/s41586-021-03235-6.
King RJ, Singh PK, Mehla K. The cholesterol pathway: impact on immunity and cancer. Trends Immunol. 2022;43:78–92. https://doi.org/10.1016/j.it.2021.11.007.
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. https://doi.org/10.1038/nature08097.
Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377-391 e9. https://doi.org/10.1016/j.ccell.2017.08.004.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8(+) T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res. 2018;6:1375–87. https://doi.org/10.1158/2326-6066.CIR-18-0095.
Li W, Guo X, Chen C, Li J. The prognostic value of arachidonic acid metabolism in breast cancer by integrated bioinformatics. Lipids Health Dis. 2022;21:103. https://doi.org/10.1186/s12944-022-01713-y.
Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–5. https://doi.org/10.1038/nature17412.
Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, et al. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol. 2021;14:187. https://doi.org/10.1186/s13045-021-01200-4.
Kleinfeld AM, Okada C. Free fatty acid release from human breast cancer tissue inhibits cytotoxic T-lymphocyte-mediated killing. J Lipid Res. 2005;46:1983–90. https://doi.org/10.1194/jlr.M500151-JLR200.
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143-156 e5. https://doi.org/10.1016/j.cmet.2019.04.002.
Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001-1012 e5. https://doi.org/10.1016/j.cmet.2021.02.015.
Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R, et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561-1577 e7. https://doi.org/10.1016/j.immuni.2021.05.003.
Liu X, Hartman CL, Li L, Albert CJ, Si F, Gao A, et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.aaz6314.
Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–97. https://doi.org/10.1146/annurev-pathol-011110-130138.
Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–55. https://doi.org/10.1038/ni.2956.
Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: from cancer progression to treatment. Prog Lipid Res. 2020;80: 101055. https://doi.org/10.1016/j.plipres.2020.101055.
Yang P, Qin H, Li Y, Xiao A, Zheng E, Zeng H, et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun. 2022;13:5782. https://doi.org/10.1038/s41467-022-33349-y.
Masetti M, Carriero R, Portale F, Marelli G, Morina N, Pandini M, et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J Exp Med. 2022. https://doi.org/10.1084/jem.20210564.
Liu Z, Gao Z, Li B, Li J, Ou Y, Yu X, et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology. 2022;11:2085432. https://doi.org/10.1080/2162402X.2022.2085432.
Timperi E, Gueguen P, Molgora M, Magagna I, Kieffer Y, Lopez-Lastra S, et al. Lipid-associated macrophages are induced by cancer-associated fibroblasts and mediate immune suppression in breast cancer. Cancer Res. 2022;82:3291–306. https://doi.org/10.1158/0008-5472.CAN-22-1427.
Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80:1438–50. https://doi.org/10.1158/0008-5472.CAN-19-2994.
Xu M, Wang X, Li Y, Geng X, Jia X, Zhang L, et al. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARgamma dependent manner. Front Immunol. 2021;12: 618501. https://doi.org/10.3389/fimmu.2021.618501.
Sag D, Cekic C, Wu R, Linden J, Hedrick CC. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun. 2015;6:6354. https://doi.org/10.1038/ncomms7354.
Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29:1376-1389 e4. https://doi.org/10.1016/j.cmet.2019.02.016.
Cheng Y, Bai F, Ren X, Sun R, Guo X, Liu W, et al. Phosphoinositide-binding protein TIPE1 promotes alternative activation of macrophages and tumor progression via PIP3/Akt/TGFbeta axis. Cancer Res. 2022;82:1603–16. https://doi.org/10.1158/0008-5472.CAN-21-0003.
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. https://doi.org/10.1038/nm.2172.
Oh DS, Lee HK. Autophagy protein ATG5 regulates CD36 expression and anti-tumor MHC class II antigen presentation in dendritic cells. Autophagy. 2019;15:2091–106. https://doi.org/10.1080/15548627.2019.1596493.
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–38. https://doi.org/10.1016/j.cell.2015.05.025.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. https://doi.org/10.1038/nri1806.
Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21:298–308. https://doi.org/10.1038/s41590-019-0589-5.
Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282-1293 e7. https://doi.org/10.1016/j.cmet.2016.12.018.
Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. https://doi.org/10.1158/0008-5472.CAN-05-0141.
Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 2020;53:187-203 e8. https://doi.org/10.1016/j.immuni.2020.06.016.
Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8(+) T cell-derived interferon-gamma. Immunity. 2019;51:381-397 e6. https://doi.org/10.1016/j.immuni.2019.06.017.
Liu J, Sun B, Guo K, Yang Z, Zhao Y, Gao M, et al. Lipid-related FABP5 activation of tumor-associated monocytes fosters immune privilege via PD-L1 expression on Treg cells in hepatocellular carcinoma. Cancer Gene Ther. 2022;29:1951–60. https://doi.org/10.1038/s41417-022-00510-0.
Kim MJ, Kim K, Park HJ, Kim GR, Hong KH, Oh JH, et al. Deletion of PD-1 destabilizes the lineage identity and metabolic fitness of tumor-infiltrating regulatory T cells. Nat Immunol. 2023;24:148–61. https://doi.org/10.1038/s41590-022-01373-1.
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015;3:1236–47. https://doi.org/10.1158/2326-6066.CIR-15-0036.
Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6: e1344804. https://doi.org/10.1080/2162402X.2017.1344804.
Yang Z, Huo Y, Zhou S, Guo J, Ma X, Li T, et al. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022;34:2018-2035 e8. https://doi.org/10.1016/j.cmet.2022.10.010.
Niavarani SR, Lawson C, Bakos O, Boudaud M, Batenchuk C, Rouleau S, et al. Lipid accumulation impairs natural killer cell cytotoxicity and tumor control in the postoperative period. BMC Cancer. 2019;19:823. https://doi.org/10.1186/s12885-019-6045-y.
Bonavita E, Bromley CP, Jonsson G, Pelly VS, Sahoo S, Walwyn-Brown K, et al. Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity. 2020;53:1215-1229 e8. https://doi.org/10.1016/j.immuni.2020.10.020.
Kobayashi T, Lam PY, Jiang H, Bednarska K, Gloury R, Murigneux V, et al. Increased lipid metabolism impairs NK cell function and mediates adaptation to the lymphoma environment. Blood. 2020;136:3004–17. https://doi.org/10.1182/blood.2020005602.
Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611–20. https://doi.org/10.3892/ol.2017.6497.
Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;38:4887–901. https://doi.org/10.1038/s41388-019-0765-y.
Li Z, Sun C, Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;11:8322–36. https://doi.org/10.7150/thno.62378.
Gong J, Lin Y, Zhang H, Liu C, Cheng Z, Yang X, et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020;11:267. https://doi.org/10.1038/s41419-020-2434-z.
Jung JG, Le A. Targeting metabolic cross talk between cancer cells and cancer-associated fibroblasts. Adv Exp Med Biol. 2021;1311:205–14. https://doi.org/10.1007/978-3-030-65768-0_15.
Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 2019;9:617–27. https://doi.org/10.1158/2159-8290.CD-18-1212.
Nambiar M, Raghavan SC. How does DNA break during chromosomal translocations? Nucleic Acids Res. 2011;39:5813–25. https://doi.org/10.1093/nar/gkr223.
Chuang HY, Lee YP, Lin WC, Lin YH, Hwang JJ. Fatty acid inhibition sensitizes androgen-dependent and -independent prostate cancer to radiotherapy via FASN/NF-kappaB pathway. Sci Rep. 2019;9:13284. https://doi.org/10.1038/s41598-019-49486-2.
Chen J, Zhang F, Ren X, Wang Y, Huang W, Zhang J, et al. Targeting fatty acid synthase sensitizes human nasopharyngeal carcinoma cells to radiation via downregulating frizzled class receptor 10. Cancer Biol Med. 2020;17:740–52. https://doi.org/10.20892/j.issn.2095-3941.2020.0219.
Jin Y, Chen Z, Dong J, Wang B, Fan S, Yang X, et al. SREBP1/FASN/cholesterol axis facilitates radioresistance in colorectal cancer. FEBS Open Bio. 2021;11:1343–52. https://doi.org/10.1002/2211-5463.13137.
Joshi PR, Zierz S. Muscle carnitine palmitoyltransferase II (CPT II) deficiency: a conceptual approach. Molecules. 2020. https://doi.org/10.3390/molecules25081784.
Tan Z, Xiao L, Tang M, Bai F, Li J, Li L, et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8:2329–47. https://doi.org/10.7150/thno.21451.
Han S, Wei R, Zhang X, Jiang N, Fan M, Huang JH, et al. CPT1A/2-mediated FAO enhancement—a metabolic target in radioresistant breast cancer. Front Oncol. 2019;9:1201. https://doi.org/10.3389/fonc.2019.01201.
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y, et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13:1511. https://doi.org/10.1038/s41467-022-29137-3.
Nix P, Lind M, Greenman J, Stafford N, Cawkwell L. Expression of Cox-2 protein in radioresistant laryngeal cancer. Ann Oncol. 2004;15:797–801. https://doi.org/10.1093/annonc/mdh185.
Cook PJ, Thomas R, Kingsley PJ, Shimizu F, Montrose DC, Marnett LJ, et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18:1379–89. https://doi.org/10.1093/neuonc/now049.
Brocard E, Oizel K, Lalier L, Pecqueur C, Paris F, Vallette FM, et al. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget. 2015;6:6840–9. https://doi.org/10.18632/oncotarget.3160.
Zhang P, Song E, Jiang M, Song Y. Celecoxib and afatinib synergistic enhance radiotherapy sensitivity on human non-small cell lung cancer A549 cells. Int J Radiat Biol. 2021;97:170–8. https://doi.org/10.1080/09553002.2021.1846817.
Cai F, Sorg O, Granci V, Lecumberri E, Miralbell R, Dupertuis YM, et al. Interaction of omega-3 polyunsaturated fatty acids with radiation therapy in two different colorectal cancer cell lines. Clin Nutr. 2014;33:164–70. https://doi.org/10.1016/j.clnu.2013.04.005.
Antal O, Hackler L Jr, Shen J, Man I, Hideghety K, Kitajka K, et al. Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: cellular, biochemical and gene expression analysis. Lipids Health Dis. 2014;13:142. https://doi.org/10.1186/1476-511X-13-142.
Zand H, Rahimipour A, Salimi S, Shafiee SM. Docosahexaenoic acid sensitizes Ramos cells to gamma-irradiation-induced apoptosis through involvement of PPAR-gamma activation and NF-kappaB suppression. Mol Cell Biochem. 2008;317:113–20. https://doi.org/10.1007/s11010-008-9838-x.
Lee H, To NB, Kim M, Nguyen YT, Cho SK, Choi HK. Metabolic and lipidomic characterization of radioresistant MDA-MB-231 human breast cancer cells to investigate potential therapeutic targets. J Pharm Biomed Anal. 2022;208: 114449. https://doi.org/10.1016/j.jpba.2021.114449.
Fang Y, Zhan Y, Xie Y, Du S, Chen Y, Zeng Z, et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC. Hepatology. 2022;75:1386–401. https://doi.org/10.1002/hep.32177.
Chmura SJ, Nodzenski E, Beckett MA, Kufe DW, Quintans J, Weichselbaum RR. Loss of ceramide production confers resistance to radiation-induced apoptosis. Cancer Res. 1997;57:1270–5.
Doan NB, Nguyen HS, Al-Gizawiy MM, Mueller WM, Sabbadini RA, Rand SD, et al. Acid ceramidase confers radioresistance to glioblastoma cells. Oncol Rep. 2017;38:1932–40. https://doi.org/10.3892/or.2017.5855.
Kumar A, Oskouian B, Fyrst H, Zhang M, Paris F, Saba JD. S1P lyase regulates DNA damage responses through a novel sphingolipid feedback mechanism. Cell Death Dis. 2011;2: e119. https://doi.org/10.1038/cddis.2011.3.
Michael JM, Lavin MF, Watters DJ. Resistance to radiation-induced apoptosis in Burkitt’s lymphoma cells is associated with defective ceramide signaling. Cancer Res. 1997;57:3600–5.
Sautin Y, Takamura N, Shklyaev S, Nagayama Y, Ohtsuru A, Namba H, et al. Ceramide-induced apoptosis of human thyroid cancer cells resistant to apoptosis by irradiation. Thyroid. 2000;10:733–40. https://doi.org/10.1089/thy.2000.10.733.
Cheng JC, Bai A, Beckham TH, Marrison ST, Yount CL, Young K, et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J Clin Invest. 2013;123:4344–58. https://doi.org/10.1172/JCI64791.
Yura Y, Masui A, Hamada M. Inhibitors of ceramide- and sphingosine-metabolizing enzymes as sensitizers in radiotherapy and chemotherapy for head and neck squamous cell carcinoma. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12082062.
Souchek JJ, Baine MJ, Lin C, Rachagani S, Gupta S, Kaur S, et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br J Cancer. 2014;111:1139–49. https://doi.org/10.1038/bjc.2014.385.
Lingwood D, Kaiser HJ, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–60. https://doi.org/10.1042/Bst0370955.
Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. https://doi.org/10.1038/35036052.
Bionda C, Athias A, Poncet D, Alphonse G, Guezguez A, Gambert P, et al. Differential regulation of cell death in head and neck cell carcinoma through alteration of cholesterol levels in lipid rafts microdomains. Biochem Pharmacol. 2008;75:761–72. https://doi.org/10.1016/j.bcp.2007.10.004.
Tavori H, Rashid S, Fazio S. On the function and homeostasis of PCSK9: reciprocal interaction with LDLR and additional lipid effects. Atherosclerosis. 2015;238:264–70. https://doi.org/10.1016/j.atherosclerosis.2014.12.017.
Gan SS, Ye JQ, Wang L, Qu FJ, Chu CM, Tian YJ, et al. Inhibition of PCSK9 protects against radiation-induced damage of prostate cancer cells. Onco Targets Therapy. 2017;10:2139–46. https://doi.org/10.2147/OTT.S129413.
Efimova EV, Ricco N, Labay E, Mauceri HJ, Flor AC, Ramamurthy A, et al. HMG-CoA reductase inhibition delays DNA repair and promotes senescence after tumor irradiation. Mol Cancer Ther. 2018;17:407–18. https://doi.org/10.1158/1535-7163.Mct-17-0288.
Mohapatra D, Das B, Suresh V, Parida D, Minz AP, Nayak U, et al. Fluvastatin sensitizes pancreatic cancer cells toward radiation therapy and suppresses radiation- and/or TGF-beta-induced tumor-associated fibrosis. Lab Invest. 2022;102:298–311. https://doi.org/10.1038/s41374-021-00690-7.
He Z, Yuan J, Shen F, Zeng F, Qi P, Wang Z, et al. Atorvastatin enhances effects of radiotherapy on prostate cancer cells and xenograft tumor mice through triggering interaction between Bcl-2 and MSH2. Med Sci Monit. 2020;26: e923560. https://doi.org/10.12659/MSM.923560.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. https://doi.org/10.1016/j.cell.2012.03.042.
Yuan H, Li XM, Zhang XY, Kang R, Tang DL. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43. https://doi.org/10.1016/j.bbrc.2016.08.124.
Lei G, Zhang YL, Koppula P, Liu XG, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62. https://doi.org/10.1038/s41422-019-0263-3.
Xie L, Song XR, Yu JM, Guo W, Wei L, Liu YL, et al. Solute carrier protein family may involve in radiation-induced radioresistance of non-small cell lung cancer. J Cancer Res Clin Oncol. 2011;137:1739–47. https://doi.org/10.1007/s00432-011-1050-9.
Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180-U120. https://doi.org/10.1038/ncb3064.
Lang XT, Green MD, Wang WM, Yu JL, Choi JE, Jiang L, et al. Radiotherapy and Immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–85. https://doi.org/10.1158/2159-8290.Cd-19-0338.
Nagane M, Kanai E, Shibata Y, Shimizu T, Yoshioka C, Maruo T, et al. Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS ONE. 2018;13: e0195151. https://doi.org/10.1371/journal.pone.0195151.
Cobler L, Zhang H, Suri P, Park C, Timmerman LA. xCT inhibition sensitizes tumors to gamma-radiation via glutathione reduction. Oncotarget. 2018;9:32280–97. https://doi.org/10.18632/oncotarget.25794.
Lei G, Zhang YL, Hong T, Zhang XD, Liu XG, Mao C, et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene. 2021;40:3533–47. https://doi.org/10.1038/s41388-021-01790-w.
Shen D, Luo J, Chen L, Ma W, Mao X, Zhang Y, et al. PARPi treatment enhances radiotherapy-induced ferroptosis and antitumor immune responses via the cGAS signaling pathway in colorectal cancer. Cancer Lett. 2022;550: 215919. https://doi.org/10.1016/j.canlet.2022.215919.
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang K, et al. Holo-lactoferrin: the link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics. 2021;11:3167–82. https://doi.org/10.7150/thno.52028.
Chen Q, Zheng W, Guan J, Liu H, Dan Y, Zhu L, et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023;30:137–51. https://doi.org/10.1038/s41418-022-01051-7.
Lin L, Wang S, Deng H, Yang W, Rao L, Tian R, et al. Endogenous labile iron pool-mediated free radical generation for cancer chemodynamic therapy. J Am Chem Soc. 2020;142:15320–30. https://doi.org/10.1021/jacs.0c05604.
Ali MY, Oliva CR, Flor S, Goswami PC, Griguer CE. Cytochrome c oxidase mediates labile iron level and radioresistance in glioblastoma. Free Radic Biol Med. 2022;185:25–35. https://doi.org/10.1016/j.freeradbiomed.2022.04.012.
Rae C, Fragkoulis GI, Chalmers AJ. Cytotoxicity and radiosensitizing activity of the fatty acid synthase inhibitor C75 is enhanced by blocking fatty acid uptake in prostate cancer cells. Adv Radiat Oncol. 2020;5:994–1005. https://doi.org/10.1016/j.adro.2020.06.022.
Lovey J, Nie D, Tovari J, Kenessey I, Timar J, Kandouz M, et al. Radiosensitivity of human prostate cancer cells can be modulated by inhibition of 12-lipoxygenase. Cancer Lett. 2013;335:495–501. https://doi.org/10.1016/j.canlet.2013.03.012.
Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15:469–84. https://doi.org/10.1021/acschembio.9b00939.
Sleire L, Skeie BS, Netland IA, Forde HE, Dodoo E, Selheim F, et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene. 2015;34:5951–9. https://doi.org/10.1038/onc.2015.60.
Zhang P, Lo A, Huang Y, Huang G, Liang G, Mott J, et al. Identification of genetic loci that control mammary tumor susceptibility through the host microenvironment. Sci Rep. 2015;5:8919. https://doi.org/10.1038/srep08919.
Ketteler J, Wittka A, Leonetti D, Roy VV, Estephan H, Maier P, et al. Caveolin-1 regulates the ASMase/ceramide-mediated radiation response of endothelial cells in the context of tumor-stroma interactions. Cell Death Dis. 2020;11:228. https://doi.org/10.1038/s41419-020-2418-z.
Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–83. https://doi.org/10.1080/15548627.2020.1714209.
Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J, et al. Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming. Sci Adv. 2020;6: eaay9789. https://doi.org/10.1126/sciadv.aay9789.
Tabraue C, Lara PC, De Mirecki-Garrido M, De La Rosa JV, Lopez-Blanco F, Fernandez-Perez L, et al. LXR signaling regulates macrophage survival and inflammation in response to ionizing radiation. Int J Radiat Oncol Biol Phys. 2019;104:913–23. https://doi.org/10.1016/j.ijrobp.2019.03.028.
Teresa Pinto A, Laranjeiro Pinto M, Patricia Cardoso A, Monteiro C, Teixeira Pinto M, Filipe Maia A, et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep. 2016;6:18765. https://doi.org/10.1038/srep18765.
Budhu S, Giese R, Gupta A, Fitzgerald K, Zappasodi R, Schad S, et al. Targeting phosphatidylserine enhances the anti-tumor response to tumor-directed radiation therapy in a preclinical model of melanoma. Cell Rep. 2021;34: 108620. https://doi.org/10.1016/j.celrep.2020.108620.
Gao F, Liu C, Guo J, Sun W, Xian L, Bai D, et al. Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer. Sci Rep. 2015;5:9613. https://doi.org/10.1038/srep09613.
Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci USA. 2017;114:1117–22. https://doi.org/10.1073/pnas.1612920114.
Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24. https://doi.org/10.1038/nri.2017.142.
Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. https://doi.org/10.1038/s41586-019-1170-y.
Wang NH, Lei Z, Yang HN, Tang Z, Yang MQ, Wang Y, et al. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: a narrative review. Ann Transl Med. 2022;10:1406. https://doi.org/10.21037/atm-22-6049.
Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. 2018;24:5368–80. https://doi.org/10.1158/1078-0432.CCR-18-1038.
Oweida AJ, Darragh L, Phan A, Binder D, Bhatia S, Mueller A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019;111:1339–49. https://doi.org/10.1093/jnci/djz036.
Altwairgi AK, Alghareeb WA, AlNajjar FH, Alhussain H, Alsaeed E, Balbaid AAO, et al. Atorvastatin in combination with radiotherapy and temozolomide for glioblastoma: a prospective phase II study. Invest New Drugs. 2021;39:226–31. https://doi.org/10.1007/s10637-020-00992-5.
Liao Z, Komaki R, Milas L, Yuan C, Kies M, Chang JY, et al. A phase I clinical trial of thoracic radiotherapy and concurrent celecoxib for patients with unfavorable performance status inoperable/unresectable non-small cell lung cancer. Clin Cancer Res. 2005;11:3342–8. https://doi.org/10.1158/1078-0432.CCR-04-1741.
Bi N, Liang J, Zhou Z, Chen D, Fu Z, Yang X, et al. Effect of concurrent chemoradiation with celecoxib vs concurrent chemoradiation alone on survival among patients with non-small cell lung cancer with and without cyclooxygenase 2 genetic variants: a phase 2 randomized clinical trial. JAMA Netw Open. 2019;2: e1918070. https://doi.org/10.1001/jamanetworkopen.2019.18070.
Takhar H, Singhal N, Mislang A, Kumar R, Kim L, Selva-Nayagam S, et al. Phase II study of celecoxib with docetaxel chemoradiotherapy followed by consolidation chemotherapy docetaxel plus cisplatin with maintenance celecoxib in inoperable stage III nonsmall cell lung cancer. Asia Pac J Clin Oncol. 2018;14:91–100. https://doi.org/10.1111/ajco.12749.
Cleary JM, Mamon HJ, Szymonifka J, Bueno R, Choi N, Donahue DM, et al. Neoadjuvant irinotecan, cisplatin, and concurrent radiation therapy with celecoxib for patients with locally advanced esophageal cancer. BMC Cancer. 2016;16:468. https://doi.org/10.1186/s12885-016-2485-9.
Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24:2723–8. https://doi.org/10.1200/JCO.2005.03.7804.
Weppelmann B, Monkemeier D. The influence of prostaglandin antagonists on radiation therapy of carcinoma of the cervix. Gynecol Oncol. 1984;17:196–9. https://doi.org/10.1016/0090-8258(84)90077-5.
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 82102201, 81874222), and the Key R & D program of Hubei Province (Grant no. 2020BCA068).
Author information
Authors and Affiliations
Contributions
DA: Investigation, Software, Visualization, Writing—original draft. DZ: Investigation, Visualization. CW: Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing—review and editing. KY: Funding acquisition, Project administration, Supervision, Validation, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval and consent to participate
The manuscript does not contain clinical studies or patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, D., Zhai, D., Wan, C. et al. The role of lipid metabolism in cancer radioresistance. Clin Transl Oncol 25, 2332–2349 (2023). https://doi.org/10.1007/s12094-023-03134-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03134-4