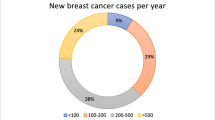
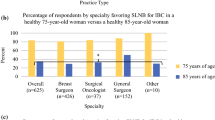
Abstract
Introduction
Given the high rate of complete nodal response, the role of axillary lymph node dissection on staging the axilla has been questioned. This survey, addressed to breast cancer surgeons in Spain, has the objective of assessing current clinical trends on axillary staging of cN + patients treated with NAC.
Methods
An online survey was conducted among breast surgeons from the Spanish Society of Surgery (AEC), Spanish Surgical Oncology Society (SEOQ), Spanish Breast Cancer Surgeons Society (AECIMA) and Spanish Gynecology and Obstetrics Society (SEGO). It was structured in 5 sections: general information and clinical practice, knowledge of clinical trials, diagnosis work-up and nodal marking, axillary staging, and axillary treatment.
Results
150 breast cancer surgeons completed the full survey (96.7%). 81.8% of respondents performed SLNB or targeted axillary dissection in cN1 patients treated with NAC. Radiological axillary response was the preferred parameter guiding the surgical strategy. The excision of the clipped node (92.0%), use of dual tracer (73.2%), and axillary US (65.9%) after treatment were the most important variables considered by respondents, to increase the accuracy of SLNB in cN + patients.
Conclusion
This survey confirms a trend toward a less invasive approach for axillary staging in cN + patients treated with NAC among breast cancer surgeons in Spain. While there is widespread agreement in less invasive approaches to axillary staging, there is, however, a lack of consensus around treatment strategy. Further, it shows a wide heterogeneity in their clinical practice. This study highlights the need for clear evidence concerning less invasive staging procedures and their oncological safety, to ensure consistent recommendations in surgical practice.



Similar content being viewed by others
Abbreviations
- NAC:
-
Neoadjuvant systemic therapy
- cN + :
-
Clinical node positive
- AEC:
-
Asociación española de Cirujanos
- SEOQ:
-
Sociedad española de Oncología Quirúrgica
- AECIMA:
-
Asociación española de cirujanos de mama
- SEGO:
-
Sociedad Española de Ginecología y Obstetricia
- SLNB:
-
Sentinel lymph node biopsy
- US:
-
Ultrasound
- ALND:
-
Axillary lymph node dissection
- HER2:
-
Human epidermal growth factor receptor 2
- TLNB:
-
Targeted lymph node biopsy
- TAD:
-
Targeted axillary dissection
- FNR:
-
False-negative rate
- MRI:
-
Magnetic resonance imaging
- PET TC:
-
Positron emission tomography
- I125:
-
Iodine-125
- ITC:
-
Isolated tumor cells
- RT:
-
Radiotherapy
- pCR:
-
Complete pathological response
- SN:
-
Sentinel node
- IHQ:
-
Immunohistochemistry
- OSNA:
-
One-step nucleic acid amplification
- LRNI:
-
Locoregional nodal irradiation
- WBRT:
-
Whole breast radiotherapy
References
Mougalian SS, Soulos PR, Killelea BK, Lannin DR, Abu-Khalaf MM, Digiovanna MP, et al. Use of neoadjuvant chemotherapy for patients with stage i to III breast cancer in the United States. Cancer. 2015;121(15):2544–52.
Vugts G, Maaskant-Braat AJG, Nieuwenhuijzen GAP, Roumen RMH, Luiten EJT, Voogd AC. Patterns of care in the administration of neo-adjuvant chemotherapy for breast cancer. Popul Based Study Breast J. 2016;22(3):316–21.
Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23(36):9304–11.
Lim B. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: Findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Vol. 26, Breast Diseases. Academic Press Inc.; 2015. p. 249–50.
Dominici LS, Negron Gonzalez VM, Buzdar AU, Lucci A, Mittendorf EA, Le-Petross HT, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116(12):2884–9.
Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen international consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz235.
Banys-paluchowski M, Gasparri ML, de Boniface J, Gentilini O, Stickeler E, Hartmann S, et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: Current status, knowledge gaps, and rationale for the eubreast-03 axsana study. Cancers (Basel). 2021;13(7):1565.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA J Am Med Assoc. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicenter cohort study. Lancet Oncol. 2013;14(7):609–18.
Boileau JF, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–63.
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patientswith node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Kuemmel S, Heil J, Rueland A, Seiberling C, Harrach H, Schindowski D, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2022;276(5):e553–62.
Siso C, de Torres J, Esgueva-Colmenarejo A, Espinosa-Bravo M, Rus N, Cordoba O, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA Trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25(3):784–91.
Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients the mari procedure. Ann Surg. 2015;261(2):378–82.
van Nijnatten TJA, Simons JM, Smidt ML, van der Pol CC, van Diest PJ, Jager A, et al. A novel less-invasive approach for axillary staging after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer by combining radioactive iodine seed localization in the axilla with the sentinel node procedure (RISAS): a Dutch pros. Clin Breast Cancer. 2017. https://doi.org/10.1016/j.clbc.2017.04.006.
Caudle AS, Bedrosian I, Milton DR, DeSnyder SM, Kuerer HM, Hunt KK, et al. Use of sentinel lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of american society of breast surgeons members. Ann Surg Oncol. 2017;24(10):2925–34.
Simons JM, Maaskant-Braat AJG, Luiten EJT, Leidenius MHK, van Nijnatten TJA, Boelens PG, et al. Patterns of axillary staging and management in clinically node positive breast cancer patients treated with neoadjuvant systemic therapy: Results of a survey amongst breast cancer specialists. Europ J Surg Oncol. 2020. https://doi.org/10.1016/j.ejso.2019.08.012.
Simons JM, Koppert LB, Luiten EJT, van der Pol CC, Samiei S, de Wilt JHW, et al. De-escalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treatment. 2020. https://doi.org/10.1007/s10549-020-05589-3.
Fisher CS, Margenthaler JA, Hunt KK, Schwartz T. The landmark series: axillary management in breast cancer. Ann Surg Oncol. 2020. https://doi.org/10.1245/s10434-019-08154-5.
Boughey JC, Alvarado MD, Lancaster RB, Fraser Symmans W, Mukhtar R, Wong JM, et al. Surgical Standards for Management of the Axilla in Breast Cancer Clinical Trials with Pathological Complete Response Endpoint. Vol. 4, npj Breast Cancer. Nature Publishing Group; 2018 Aug 17;4:26. https://doi.org/10.1038/s41523-018-0074-6.
Kirkilesis G, Constantinidou A, Kontos M. False negativity of targeted axillary dissection in breast cancer. Breast Care. 2021;16(5):532–8.
Banys-Paluchowski M, Gruber IV, Hartkopf A, Paluchowski P, Krawczyk N, Marx M, et al. Axillary ultrasound for prediction of response to neoadjuvant therapy in the context of surgical strategies to axillary dissection in primary breast cancer: a systematic review of the current literature. Arch Gynecol Obstet. 2020. https://doi.org/10.1007/s00404-019-05428-x.
Kantor O, Sipsy LMN, Yao K, James TA. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018. https://doi.org/10.1245/s10434-018-6345-5.
Fowble BL, Einck JP, Kim DN, McCloskey S, Mayadev J, Yashar C, et al. Role of postmastectomy radiation after neoadjuvant chemotherapy in stage II-III breast cancer. Vol. 83, International Journal of Radiation Oncology Biology Physics. 2012 Jun 1;83(2):494-503. https://doi.org/10.1016/j.ijrobp.2012.01.068.
White J, Mamounas E. Locoregional radiotherapy in patients with breast cancer responding to neoadjuvant chemotherapy: A paradigm for treatment individualization. Vol. 32, Journal of Clinical Oncology. American Society of Clinical Oncology; 2014 Feb 20;32(6):494-5. https://doi.org/10.1200/JCO.2013.53.4974.
Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J Clin Oncol. 2012;30(32):3960–6.
Morrow M, Khan AJ. SPECIAL SERIES: LOCOREGIONAL MANAGEMENT OF BREAST CANCER review articles Locoregional Management After Neoadjuvant Chemotherapy [Internet]. 2020.
Kantor O, Wong S, Weiss A, Metzger O, Mittendorf EA, King TA. Prognostic significance of residual nodal disease after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer. npj Breast Cancer. 2020. https://doi.org/10.1038/s41523-020-00177-6.
Viale G, Fusco N. Pathology after neoadjuvant treatment - How to assess residual disease. Breast. 2022 Mar;62 Suppl 1(Suppl 1):S25-S28. https://doi.org/10.1016/j.breast.2021.11.009.
Moo TA, Edelweiss M, Hajiyeva S, Stempel M, Raiss M, Zabor EC, et al. Is low-volume disease in the sentinel node after neoadjuvant chemotherapy an indication for axillary dissection? Ann Surg Oncol. 2018;25(6):1488–94.
Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, Vicini E, Morigi C, Corso G, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021. https://doi.org/10.1016/j.ejso.2020.10.014.
Piltin MA, Hoskin TL, Day CN, Davis J, Boughey JC. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27(12):4795–801.
Funding
Non-financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval and Informed consent
This study has been approved by the research ethics committee – institutional review board- of Ramon y Cajal Hospital (Madrid).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Munoz, P., Corral, S., Martínez-Regueira, F. et al. Axillary staging and management of cN + breast cancer patients treated with neoadjuvant chemotherapy: results of a survey among breast cancer surgeons in Spain. Clin Transl Oncol 25, 1463–1471 (2023). https://doi.org/10.1007/s12094-022-03049-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03049-6