Abstract
Purpose
Although outcomes of children with acute myeloid leukemia (AML) have improved over the last decades, around one-third of patients relapse. Measurable (or minimal) residual disease (MRD) monitoring may guide therapy adjustments or pre-emptive treatments before overt hematological relapse.
Methods
In this study, we review 297 bone marrow samples from 20 real-life pediatric AML patients using three MRD monitoring methods: multiparametric flow cytometry (MFC), fluorescent in situ hybridization (FISH) and polymerase chain reaction (PCR).
Results
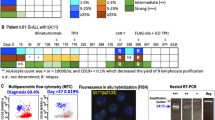
Patients showed a 3-year overall survival of 73% and a 3-year event-free survival of 68%. Global relapse rate was of 25%. All relapses were preceded by the reappearance of MRD detection by: (1) MFC (p = 0.001), (2) PCR and/or FISH in patients with an identifiable chromosomal translocation (p = 0.03) and/or (3) one log increase of Wilms tumor gene 1 (WT1) expression in two consecutive samples (p = 0.02). The median times from MRD detection to relapse were 26, 111, and 140 days for MFC, specific PCR and FISH, and a one log increment of WT1, respectively.
Conclusions
MFC, FISH and PCR are complementary methods that can anticipate relapse of childhood AML by weeks to several months. However, in our series, pre-emptive therapies were not able to prevent disease progression. Therefore, more sensitive MRD monitoring methods that further anticipate relapse and more effective pre-emptive therapies are needed.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Elgarten CW, Aplenc R. Pediatric acute myeloid leukemia: updates on biology, risk stratification, and therapy. Curr Opin Pediatr. 2020;32(1):57–66.
Langebrake C, Creutzig U, Dworzak M, Hrusak O, Mejstrikova E, Griesinger F, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J Clin Oncol. 2006;24(22):3686–92.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52.
Tierens A, Bjørklund E, Siitonen S, Marquart HV, Wulff-Juergensen G, Pelliniemi TT, et al. Residual disease detected by flow cytometry in an independent predictor of survival in childhood acute myeloid leukemia; results of the NOPHO-AML 2004 study. Br J Haematol. 2016;174(4):600–9.
Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very high risk leukemia. Blood. 2012;120(2):468–72.
Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102(5):865–73.
Benetton M, Merli P, Walter C, Hansen M, Da Ros A, Polato K, et al. Molecular measurable residual disease assessment before hematopoietic stem cell transplantation in pediatric acute myeloid leukemia patients: a retrospective study by the I-BFM study group. Biomedicines. 2022;10(7):1530.
Sievers EL, Lange BJ, Buckley JD, Smith FO, Wells DA, Daigneault-Creech CA, et al. Prediction of relapse of pediatric acute myeloid leukemia by use of flow multidimensional flow cytometry. J Natl Cancer Inst. 1996;88(20):1483–8.
Ommen HS. Monitoring minimal residual disease in acute myeloid leukaemia: a review of the current evolving strategies. Ther Adv Hematol. 2016;7(1):3–16.
Kern W, Bacher U, Haferlach C, Schnittger S, Haferlach T. The role of multiparameter flow cytometry for disease monitoring in AML. Best Pract Res Clin Haematol. 2010;23(3):379–90.
Terwijn M, van Putten WLJ, Kelder A, van der Velderi VHJ, Brooimans RA, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889–97.
Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonák J, Lind K, et al. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125.
Ehinger M, Petterson L. Measurable residual disease testing for personalized treatment of acute myeloid leukemia. APMIS. 2019;127(5):337–51.
Vettenranta K, Autio K, Hovi L, Knuutila S, Saarinen-Pihkala UM. Follow-up of minimal residual disease in pediatric acute myeloblastic leukemia using metaphase-FISH. Leuk Lymphoma. 2002;43(6):1261–5.
Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DKH, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom medical research council treatment trials AML 10 and 12. J Clin Oncol. 2010;28(16):2674–81.
Von Neuhoff C, Reinhardt D, Sander A, Zimmermann M, Bradtke J, Betts DR, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. 2010;28(16):2682–9.
Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Rennevile A, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32(2):273–84.
Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489–96.
Zhang H, Zhang L, Li Y, Gu H, Wang X. SET-CAN fusion gene in leukemia and myeloid neoplasms: report of three cases and a literature review. Onco Targets Ter. 2020;13:7665–81.
Ozbek U, Kandilci A, van Baal S, Bonten J, Boyd K, Franken P, et al. SET-CAN, the product of the t(9;9) in acute undifferentiated leukemia, causes expansion of early hematopoietic progenitors and hyperproliferation of stomach mucosa in transgenic mice. Am J Pathol. 2007;171(2):654–66.
Menssen HD, Renkl H-J, Rodeck U, Maurer J, Notter M, Schwartz S, et al. Presence of Wilms’ tumor gene (wt1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia. 1995;9(6):1060–7.
Cilloni D, Gottardi E, De Micheli D, Serra A, Volpe G, Messa F, et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia. 2002;16(10):2115–21.
Pozzi S, Geroldi S, Tedone E, Luchetti S, Grasso R, Colombo N, et al. Leukemia relapse after allogeneic transplants for acute myeloid leukaemia: predictive role of WT1 expression. Br J Haematol. 2013;160(4):503–9.
Rossi G, Minervini MM, Carella AM, de Waure C, di Nardo F, Melillo L, et al. Comparison between multiparameter flow cytometry and WT1-RNA quantification in monitoring minimal residual disease in acute myeloid leukemia without specific molecular targets. Leuk Res. 2012;36(4):401–6.
Patriarca F, Sperotto A, Lorentino F, Oldani E, Mammoliti S, Isola M, et al. Donor lymphocyte infusions after allogeneic stem cell transplantation in acute leukemia: a survey from the Gruppo italiano trapianto midollo osseo (GITMO). Front Oncol. 2020;10: 572918.
Platzbecker U, Middeke JM, Socke K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azatidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA 2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19(12):1668–79.
Elmaagacli AH, Beelen DW, Trenn G, Schmidt O, Nahler M, Schaefer UW. Induction of a graft-versus-leukemia reaction by cyclosporin A withdrawal as immunotherapy for leukemia relapsing after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23:771–7.
Guo M, Hu KX, Liu GX, Yu CL, Qiao JH, Sun QY, et al. HLA-mismatched stem-cell microtransplatation as postremission therapy for acute myeloid leukemia: long-term follow up. J Clin Oncol. 2012;30(33):4084–90.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. Classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2008.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2017.
Minguela A, Vasco-Mogorrón MA, Campillo JA, Cabañas V, Remigia MJ, Berenguer M, et al. Predictive value of 1q21 gain in multiple myeloma is strongly dependent on concurrent cytogenetic abnormalities and first-line treatment. Am J Cancer Res. 2021;11(9):4438–54.
Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD working party. Blood. 2018;131(12):1275–91.
Miyamoto T, Nagafuji K, Akashi K, Harada M, Kyo T, Akashi T, et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia. Blood. 1996;87(11):4789–96.
Hatlen M, Wang L, Nimer S. AML1-ETO driven acute leukemia: insights into pathogenesis and potential therapeutic approaches. Front Med. 2012;6(3):248–62.
Matsuo H, Iijima-Yamashita Y, Yamada M, Deguchi T, Kiyokawa N, Shimada A, et al. Monitoring of fusion gene transcripts to predict relapse in pediatric acute myeloid leukemia. Pediatr Int. 2018;60(1):41–6.
Juul-Dam KL, Ommen HB, Nyvold CG, Walter C, Vålerhugen H, Kairisto V, et al. Measurable residual disease assessment by qPCR in peripheral blood is an informative tool for disease surveillance in childhood acute myeloid leukaemia. Br J Haematol. 2020;190(2):198–208.
Maurer U, Brieger J, Weidmann E, Mitrou PS, Hoelzer D, Bergmann L. The Wilms’ tumor gene is expressed in a subset of CD34+ progenitors and downregulated early in the course of differentiation in vitro. Exp Hematol. 1997;25(9):945–50.
Weisser M, Kern W, Rauhut S, Schoch C, Hiddemann W, Haferlach T, et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia. 2005;19(8):1416–23.
Mashima K, Oh I, Ikeda T, Toda Y, Ito S, Umino K, et al. Role of sequential monitoring of WT1 gene expression in patients with acute myeloid leukemia for the early detection of leukemia relapse. Clin Lymphoma Myeloma Leuk. 2018;18(12):521–7.
Acknowledgements
We would like to acknowledge the hematologists involved in the bone marrow transplantation of patients Drs. José María Moraleda, Andrés Sánchez-Salinas, Joaquín Gómez-Espuch and Jorge Montserrat Coll, nurses and other healthcare members from the pediatric hematology and oncology department, laboratory technicians María Carmen Martínez Solano and María Dolores García Arnao, and especially to all patients and families.
Funding
The researcher M.V.M.S. was funded by Asociación Pablo Ugarte (APU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
The study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients (and/or their parents) in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramos Elbal, E., Fuster, J.L., Campillo, J. et al. Measurable residual disease study through three different methods can anticipate relapse and guide pre-emptive therapy in childhood acute myeloid leukemia. Clin Transl Oncol 25, 1446–1454 (2023). https://doi.org/10.1007/s12094-022-03042-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03042-z