Abstract
Background
Natural killer (NK) cells, as professional cytotoxic cells, play a key role against cancer in the early and metastatic stages. Their functional defects are highly associated with the initiation or progression of breast cancer (BC). Here, we investigated the phenotypic characterization of NK cells in 26 newly diagnosed BC patients in comparison to 12 healthy counterparts.
Methods
Expression of CXCR3 and PD-1, and also NKG2D, and TGF-βRII were studied on CD56dim and CD56bright NK cells from fresh peripheral blood (PB) samples using flow cytometry. The plasma levels of IFN-γ and soluble MIC-A levels were also assessed by ELISA.
Results
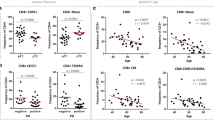
Both CD56dim and CD56bright NK subtypes showed lower CXCR3 and NKG2D expression in BC patients than healthy subjects. Furthermore, patients’ CD56bright NK cells significantly showed higher expression levels of TGF-βRII and PD-1. Interestingly, increased concentration of MIC-A level in plasma of BC patients was associated with the higher TGF-βRII and PD-1 expression in all NK cells, while the plasma level of IFN-γ was associated with the lower TGF-βRII expression on CD56bright NK cells in these patients.
Conclusion
Our results demonstrated phenotypically suppressed-NK cells, especially in the CD56bright subset of BC patients. It specifies their potential incompetence and outlines decrement of their anti-tumor activity, which could be interrelated with the tumor pathogenesis, TME immunosuppression, and so disease progression. The induction of compensatory mechanisms revives NK cells function and could be used in combination with the conventional treatments as a putative therapeutic approach for targeted immunotherapy.






Similar content being viewed by others
Abbreviations
- PB:
-
Peripheral blood
- NK cell:
-
Natural killer cell
- BC:
-
Breast cancer
- NKG2D:
-
Natural killer group 2D
- CXCR3:
-
C-X-C motif chemokine receptor 3
- PD-1:
-
Programmed death receptor-1
- TGF-βRII:
-
Transforming growth factor beta receptor II
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Xiang YJ, Fu QY, Ma ZB, Gao DZ, Zhang Q, Li YY, et al. Screening for candidate genes related to breast cancer with cDNA microarray analysis. Chronic Dis Transl Med. 2015;1(2):65–72. https://doi.org/10.1016/j.cdtm.2015.02.001.
Bates JP, Derakhshandeh R, Jones L, Webb TJ. Mechanisms of immune evasion in breast cancer. BMC Cancer. 2018;18(1):556. https://doi.org/10.1186/s12885-018-4441-3.
Arianfar E, Shahgordi S, Memarian A. Natural killer cell defects in breast cancer: a key pathway for tumor evasion. Int Rev Immunol. 2021;40(3):197–216. https://doi.org/10.1080/08830185.2020.1845670.
Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol. 2019;10:3038. https://doi.org/10.3389/fimmu.2019.03038.
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577–83. https://doi.org/10.1002/(SICI)1097-0142(20000201)88:3%3c577::AID-CNCR13%3e3.0.CO;2-V.
Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–8. https://doi.org/10.1002/(sici)1097-0142(19970615)79:12%3c2320::aid-cncr5%3e3.0.co;2-p.
Yoon SR, Kim TD, Choi I. Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med. 2015;47(2): e141. https://doi.org/10.1038/emm.2014.114.
Dezell SA, Ahn YO, Spanholtz J, Wang H, Weeres M, Jackson S, et al. Natural killer cell differentiation from hematopoietic stem cells: a comparative analysis of heparin-and stromal cell-supported methods. Biol Blood Marrow Transplant. 2012;18(4):536–45. https://doi.org/10.1016/j.bbmt.2011.11.023.
Mah AY, Cooper MA. Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol. 2016;36(2):131–47. https://doi.org/10.1615/CritRevImmunol.2016017387.
Amand M, Iserentant G, Poli A, Sleiman M, Fievez V, Sanchez IP, et al. Human CD56dimCD16dim cells as an individualized natural killer cell subset. Front Immunol. 2017;8:699. https://doi.org/10.3389/fimmu.2017.00699.
Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M, et al. Human CD56bright NK cells: an update. J Immunol. 2016;196(7):2923–31. https://doi.org/10.4049/jimmunol.1502570.
Fernández-Messina L, Reyburn HT, Valés-Gómez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012;3:299. https://doi.org/10.3389/fimmu.2012.00299.
Mistry AR, O’Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121(4):439–47. https://doi.org/10.1111/j.1365-2567.2007.02652.x.
Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–90. https://doi.org/10.1038/nri1199.
Zhao Y, Chen N, Yu Y, Zhou L, Niu C, Liu Y, et al. Prognostic value of MICA/B in cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96384–95. https://doi.org/10.18632/oncotarget.21466.
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8. https://doi.org/10.1038/85330.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331.
Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol. 2017;139(1):335-46.e3. https://doi.org/10.1016/j.jaci.2016.04.025.
Pesce S, Greppi M, Grossi F, Del Zotto G, Moretta L, Sivori S, et al. PD/1-PD-Ls checkpoint: insight on the potential role of NK cells. Front Immunol. 2019;10:1242. https://doi.org/10.3389/fimmu.2019.01242.
Beldi-Ferchiou A, Lambert M, Dogniaux S, Vély F, Vivier E, Olive D, et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. 2016;7(45):72961–77. https://doi.org/10.18632/oncotarget.12150.
Benson DM, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–94. https://doi.org/10.1182/blood-2010-02-271874.
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36(44):6143–53. https://doi.org/10.1038/onc.2017.209.
Garrod KR, Wei SH, Parker I, Cahalan MD. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc Natl Acad Sci. 2007;104(29):12081–6. https://doi.org/10.1073/pnas.0702867104.
Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68(20):8437–45. https://doi.org/10.1158/0008-5472.CAN-08-1440.
Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207–15. https://doi.org/10.1038/icb.2010.158.
Wennerberg E, Pfefferle A, Ekblad L, Yoshimoto Y, Kremer V, Kaminskyy VO, et al. Human anaplastic thyroid carcinoma cells are sensitive to NK cell-mediated lysis via ULBP2/5/6 and chemoattract NK cells. Clin Cancer Res. 2014;20(22):5733–44. https://doi.org/10.1158/1078-0432.CCR-14-0291.
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. https://doi.org/10.1146/annurev.immunol.24.021605.090737.
Slattery K, Zaiatz-Bittencourt V, Woods E, Brennan K, Marks S, Chew S, et al. TGFβ drives mitochondrial dysfunction in peripheral blood NK cells during metastatic breast cancer. Biorxiv. 2019. https://doi.org/10.1101/648501.
Allan DS, Rybalov B, Awong G, Zúñiga-Pflücker JC, Kopcow HD, Carlyle JR, et al. TGF-β affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40(8):2289–95. https://doi.org/10.1002/eji.200939910.
Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ. Serum levels of transforming growth factor beta1 in patients with breast cancer. Arch Surg. 2001;136(8):937–40. https://doi.org/10.1001/archsurg.136.8.937.
Viel S, Marçais A, Guimaraes FS, Loftus R, Rabilloud J, Grau M, et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal. 2016;9(415):ra19. https://doi.org/10.1126/scisignal.aad1884.
Mohammadi S, Ebadpour MR, Sedighi S, Saeedi M, Memarian A. Glucocorticoid-induced leucine zipper expression is associated with response to treatment and immunoregulation in systemic lupus erythematosus. Clin Rheumatol. 2017;36(8):1765–72. https://doi.org/10.1007/s10067-017-3711-9.
Ajam F, Aghaei M, Mohammadi S, Samiei H, Behnampour N, Memarian A. PD-1 expression on CD8+CD28- T cells within inflammatory synovium is associated with relapse: a cohort of rheumatoid arthritis. Immunol Lett. 2020;228:76–82. https://doi.org/10.1016/j.imlet.2020.10.005.
Caras I, Grigorescu A, Stavaru C, Radu DL, Mogos I, Szegli G, et al. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother. 2004;53(12):1146–52. https://doi.org/10.1007/s00262-004-0556-2.
Nieto-Velázquez NG, Torres-Ramos YD, Muñoz-Sánchez JL, Espinosa-Godoy L, Gómez-Cortés S, Moreno J, et al. Altered expression of natural cytotoxicity receptors and NKG2D on peripheral blood NK cell subsets in breast cancer patients. Transl Oncol. 2016;9(5):384–91. https://doi.org/10.1016/j.tranon.2016.07.003.
Mamessier E, Pradel LC, Thibult ML, Drevet C, Zouine A, Jacquemier J, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol. 2013;190(5):2424–36. https://doi.org/10.4049/jimmunol.1200140.
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609–22. https://doi.org/10.1172/JCI45816.
Ostapchuk YO, Cetin EA, Perfilyeva YV, Yilmaz A, Skiba YA, Chirkin AP, et al. Peripheral blood NK cells expressing HLA-G, IL-10 and TGF-β in healthy donors and breast cancer patients. Cell Immunol. 2015;298(1–2):37–46. https://doi.org/10.1016/j.cellimm.2015.09.002.
Gharagozloo M, Kalantari H, Rezaei A, Maracy MR, Salehi M, Bahador A, et al. The decrease in NKG2D+ natural killer cells in peripheral blood of patients with metastatic colorectal cancer. Bratisl Lek Listy. 2015;116(5):296–301. https://doi.org/10.4149/bll_2015_056.
Sun B, Yang D, Dai H, Liu X, Jia R, Cui X, et al. Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res. 2019;7(11):1813–23. https://doi.org/10.1158/2326-6066.CIR-19-0026.
Zhang Y, Li X, Zhang J, Mao L. Novel cellular immunotherapy using NKG2D CAR-T for the treatment of cervical cancer. Biomed Pharmacother. 2020;131: 110562. https://doi.org/10.1016/j.biopha.2020.110562.
Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80. https://doi.org/10.1016/j.immuni.2008.02.016.
Fuertes MB, Domaica CI, Zwirner NW. Leveraging NKG2D ligands in immuno-oncology. Front Immunol. 2021;12: 713158. https://doi.org/10.3389/fimmu.2021.713158.
Saito H, Osaki T, Ikeguchi M. Decreased NKG2D expression on NK cells correlates with impaired NK cell function in patients with gastric cancer. Gastric Cancer. 2012;15(1):27–33. https://doi.org/10.1007/s10120-011-0059-8.
Shen J, Pan J, Du C, Si W, Yao M, Xu L, et al. Silencing NKG2D ligand-targeting miRNAs enhances natural killer cell-mediated cytotoxicity in breast cancer. Cell Death Dis. 2017;8(4): e2740. https://doi.org/10.1038/cddis.2017.158.
Schmiedel D, Mandelboim O. NKG2D ligands-critical targets for cancer immune escape and therapy. Front Immunol. 2018;9:2040. https://doi.org/10.3389/fimmu.2018.02040.
de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer. 2012;12:24. https://doi.org/10.1186/1471-2407-12-24.
Molfetta R, Quatrini L, Santoni A, Paolini R. Regulation of NKG2D-dependent NK cell functions: the Yin and the Yang of receptor endocytosis. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18081677.
Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T, et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189(3):1360–71. https://doi.org/10.4049/jimmunol.1200796.
Aquino-López A, Senyukov VV, Vlasic Z, Kleinerman ES, Lee DA. Interferon gamma induces changes in natural killer (NK) cell ligand expression and alters NK cell-mediated lysis of pediatric cancer cell lines. Front Immunol. 2017;8:391. https://doi.org/10.3389/fimmu.2017.00391.
Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91(2):299–309. https://doi.org/10.1189/jlb.0611308.
Quatrini L, Mariotti FR, Munari E, Tumino N, Vacca P, Moretta L. The immune checkpoint PD-1 in natural killer cells: expression, function and targeting in tumour immunotherapy. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12113285.
Mizuno R, Sugiura D, Shimizu K, Maruhashi T, Watada M, Okazaki IM, et al. PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Front Immunol. 2019;10:630. https://doi.org/10.3389/fimmu.2019.00630.
Qiu Y, Yang Y, Yang R, Liu C, Hsu J-M, Jiang Z, et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40(31):4992–5001. https://doi.org/10.1038/s41388-021-01896-1.
Vari F, Arpon D, Keane C, Hertzberg MS, Talaulikar D, Jain S, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018;131(16):1809–19. https://doi.org/10.1182/blood-2017-07-796342.
Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. 2014;124(11):4781–94. https://doi.org/10.1172/JCI74337.
Slattery K, Woods E, Zaiatz-Bittencourt V, Marks S, Chew S, Conroy M, et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-002044.
Zaiatz-Bittencourt V, Finlay DK, Gardiner CM. Canonical TGF-β signaling pathway represses human NK cell metabolism. J Immunol. 2018;200(12):3934–41. https://doi.org/10.4049/jimmunol.1701461.
Nagaraj NS, Datta PK. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin Investig Drugs. 2010;19(1):77–91. https://doi.org/10.1517/13543780903382609.
Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100(7):4120–5. https://doi.org/10.1073/pnas.0730640100.
Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, et al. Pro-and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24(5):575–90. https://doi.org/10.1016/j.immuni.2006.03.016.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–50. https://doi.org/10.1093/jnci/djp082.
Regis S, Dondero A, Caliendo F, Bottino C, Castriconi R. NK cell function regulation by TGF-β-induced epigenetic mechanisms. Front Immunol. 2020;11:311. https://doi.org/10.3389/fimmu.2020.00311.
Riggan L, Shah S, O’Sullivan TE. Arrested development: suppression of NK cell function in the tumor microenvironment. Clin Transl Immunology. 2021;10(1): e1238. https://doi.org/10.1002/cti2.1238.
Bonanni V, Antonangeli F, Santoni A, Bernardini G. Targeting of CXCR3 improves anti-myeloma efficacy of adoptively transferred activated natural killer cells. J Immunother Cancer. 2019;7(1):290. https://doi.org/10.1186/s40425-019-0751-5.
Nersesian S, Schwartz SL, Grantham SR, MacLean LK, Lee SN, Pugh-Toole M, et al. NK cell infiltration is associated with improved overall survival in solid cancers: a systematic review and meta-analysis. Transl Oncol. 2021;14(1): 100930. https://doi.org/10.1016/j.tranon.2020.100930.
Zhang S, Liu W, Hu B, Wang P, Lv X, Chen S, et al. Prognostic significance of tumor-infiltrating natural killer cells in solid tumors: a systematic review and meta-analysis. Front Immunol. 2020;11:1242. https://doi.org/10.3389/fimmu.2020.01242.
Wuest TR, Carr DJ. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol. 2008;181(11):7985–93. https://doi.org/10.4049/jimmunol.181.11.7985.
Kajitani K, Tanaka Y, Arihiro K, Kataoka T, Ohdan H. Mechanistic analysis of the antitumor efficacy of human natural killer cells against breast cancer cells. Breast Cancer Res Treat. 2012;134(1):139–55. https://doi.org/10.1007/s10549-011-1944-x.
Ejaeidi AA, Craft BS, Puneky LV, Lewis RE, Cruse JM. Hormone receptor-independent CXCL10 production is associated with the regulation of cellular factors linked to breast cancer progression and metastasis. Exp Mol Pathol. 2015;99(1):163–72. https://doi.org/10.1016/j.yexmp.2015.06.002.
Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J Immunother. 2007;30(5):490–8. https://doi.org/10.1097/CJI.0b013e318031b551.
Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175(3):1636–42. https://doi.org/10.4049/jimmunol.175.3.1636.
Funding
This article was granted by Iran National Science Foundation (INSF) 0.13039/501100003968, with Grant No. 96010101 and Golestan University of Medical Sciences with Grant No. 110632.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was approved by the ethics committee of Golestan University of Medical Sciences (No: IR.GOUMS.REC.1398.020).
Informed consent
Written informed consent following the Declaration of Helsinki was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2022_2997_MOESM1_ESM.tif
Supplementary file1. Phenotypic analysis of TGF-βRII, NKG2D, PD-1, and CXCR3 on peripheral blood. CD56dim NK cells (A) and CD56bright NK cells (B) in BC patients. Flow cytometry data are presented as mean fluorescence intensity (MFI) of TGF-βRII+, NKG2D+, PD-1+, and CXCR3+ NK cells in 26 BC patients and 12 healthy donors. Analysis was performed using the independent sample T-test. p-values lower than 0.05 were considered statistically significant. Data of each bar demonstrates means ±SE. NS= not significant. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (TIF 4839 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arianfar, E., Khandoozi, S.R., Mohammadi, S. et al. Suppression of CD56bright NK cells in breast cancer patients is associated with the PD-1 and TGF-βRII expression. Clin Transl Oncol 25, 841–851 (2023). https://doi.org/10.1007/s12094-022-02997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02997-3