Abstract
Purpose
Both cyclic pentapeptide c(RGDfK) and acridine orange (AO) exhibit antitumor effects and cell permeability. This study aimed to evaluate the nuclear targeting efficiency and safety of the nuclear targeting probe for bladder cancer (BCa) synthesized by c(RGDfK) and AO.
Methods
The nuclear targeting probe AO-(cRGDfK)2 was synthesized from AO hydrochloride, azided c(RGDfK), and a near-infrared skeleton synthesized via click chemistry reactions. The effect of the AO-(cRGDfK)2 probe on cell viability was assessed in BCa 5637 cells. The tumor cell targeting efficacy of the AO-(cRGDfK)2 probe was evaluated in BCa cells in vitro and in tumor-bearing mice in vivo. Nuclear-specific accumulation of fluorescence probe in BCa tumor cells was evaluated using laser scanning confocal microscopy (LSCM). Hematoxylin and eosin staining was performed to detect histopathological changes in the spleen, heart, liver, and kidney.
Results
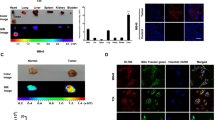
The AO-(cRGDfK)2 probe did not cause a significant reduction in cell viability. LSCM analysis showed that AO-(cRGDfK)2 exhibited nuclear-specific ambulation in BCa cells and was not accumulated in 293T cells. Also, this probe efficiently targeted tumor cells in the serum and urine samples. In vivo imaging system of tumor-bearing mice showed that ~ 80% percent of fluorescence signal was accumulated in the tumor sites. The probe did not change histopathology in the heart, liver, spleen, and kidney in tumor-bearing mice after the 21-day treatment.
Conclusions
The AO-(cRGDfK)2 probe exhibited nuclear-specific accumulation in BCa cells without cytotoxicity, which provides an innovative alternative to improve anticancer therapy for BCa.






Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Wang S-C, Chang Y-C, Wu M-Y, Yu C-Y, Chen S-L, Sung W-W. Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer. Cancers. 2021;13:3933.
van Kessel KE, van der Keur KA, Dyrskjøt L, et al. Molecular markers increase precision of the European Association of urology non–muscle-invasive bladder cancer progression risk groups. Clin Cancer Res. 2018;24:1586–93.
van den Bosch S, Witjes JA. Long-term cancer-specific survival in patients with high-risk, non–muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60:493–500.
Byvaltsev VA, Bardonova LA, Onaka NR, et al. Acridine orange: a review of novel applications for surgical cancer imaging and therapy. Front Oncol. 2019;9:925.
Kusuzaki K, Hosogi S, Ashihara E, et al. Translational research of photodynamic therapy with acridine orange which targets cancer acidity. Curr Pharm Des. 2012;18:1414–20.
Osman H, Elsahy D, Saadatzadeh MR, et al. Acridine orange as a novel photosensitizer for photodynamic therapy in glioblastoma. World Neurosurg. 2018;114:e1310–5.
Liu J, Zhang W, Kumar A, et al. Acridine orange encapsulated mesoporous manganese dioxide nanoparticles to enhance radiotherapy. Bioconjug Chem. 2019;31:82–92.
Lin Y-C, Lin J-F, Tsai T-F, et al. Acridine orange exhibits photodamage in human bladder cancer cells under blue light exposure. Sci Rep. 2017;7:1–11.
Wang D, Liu M, Wu Y, et al. Idarubicin/mithramycin-acridine orange combination drugs co-loaded by DNA nanostructures: Different effects of intercalation and groove binding on drug release and cytotoxicity. J Mol Liq. 2022;355: 118947.
Mezu-Ndubuisi OJ, Maheshwari A. The role of integrins in inflammation and angiogenesis. Pediatr Res. 2021;89:1619–26.
LaFlamme SE, Mathew-Steiner S, Singh N, Colello-Borges D, Nieves B. Integrin and microtubule crosstalk in the regulation of cellular processes. Cell Mol Life Sci. 2018;75:4177–85.
Ludwig BS, Kessler H, Kossatz S, Reuning U. RGD-binding integrins revisited: how recently discovered functions and novel synthetic ligands (re-) shape an ever-evolving field. Cancers. 2021;13:1711.
Kapp TG, Rechenmacher F, Neubauer S, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7:1–13.
Heller M, Kumar V, Pabst A, Brieger J, Al-Nawas B, Kämmerer P. Osseous response on linear and cyclic RGD-peptides immobilized on titanium surfaces in vitro and in vivo. J Biomed Mater Res A. 2018;106:419–27.
Bogdanowich-Knipp S, Jois D, Siahaan T. The effect of conformation on the solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:523–9.
Zhou D, Zhang G, Gan Z. c(RGDfK) decorated micellar drug delivery system for intravesical instilled chemotherapy of superficial bladder cancer. J Control Release. 2013;169:204–10.
Klimpel A, Lützenburg T, Neundorf I. Recent advances of anti-cancer therapies including the use of cell-penetrating peptides. Curr Opin Pharmacol. 2019;47:8–13.
Wang H, Wang Z, Chen W, et al. Self-assembly of photosensitive and radiotherapeutic peptide for combined photodynamic-radio cancer therapy with intracellular delivery of miRNA-139-5p. Bioorg Med Chem. 2021;44: 116305.
Yi Y, Kim HJ, Mi P, et al. Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. J Control Release. 2016;244:247–56.
Mao B, Liu C, Zheng W, et al. Cyclic cRGDfk peptide and Chlorin e6 functionalized silk fibroin nanoparticles for targeted drug delivery and photodynamic therapy. Biomaterials. 2018;161:306–20.
Bankó C, Nagy ZL, Nagy M, et al. Isocyanide substitution in acridine orange shifts DNA damage-mediated phototoxicity to permeabilization of the lysosomal membrane in cancer cells. Cancers. 2021;13:5652.
Lazic S, Kaspler P, Mandel A, Kulkani G, Jewett M, Lilge L. Photodynamic therapy for non-muscle invasive bladder cancer (NMIBC) mediated by instilled photosensitizer TLD1433 and green light activation. Photochem Photobiol Sci. 2016;15:481–95.
Fang L, Zhao Z, Wang J, et al. Light-controllable charge-reversal nanoparticles with polyinosinic-polycytidylic acid for enhancing immunotherapy of triple negative breast cancer. Acta Pharm Sin B. 2022;12:353–63.
Song W, Kuang J, Li C-X, et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12:1978–89.
Dijkgraaf I, Kruijtzer JAW, Frielink C, et al. Synthesis and biological evaluation of potent αvβ3-integrin receptor antagonists. Nucl Med Biol. 2006;33:953–61.
Park K-Y, Kim J. Cyclic pentapeptide cRGDfK enhances the inhibitory effect of sunitinib on TGF-β1-induced epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. PLoS ONE. 2020;15: e0232917.
Funding
This work was supported by the National Natural Science Foundation of China (82004110), the Jiangsu Province Key Research and Development Program (BE2020758, BE2019637); the Xuzhou Medical Outstanding Talents (Xuzhou Health Education Research [2017] No.22); the Jiangsu Medical Innovation Team (CXTDA2017048); the Key Projects of Xuzhou Science and Technology Plan (KC19075, KC21184) and the Xuzhou clinical medicine expert team project(2018TD004); the Jiangxi Province Health Commission Science and technology project (202120104).
Author information
Authors and Affiliations
Contributions
JQ, QL, GW, CH and ZS are responsible for the conception or design of the work. JQ, QL, GW, LH, XL, XW, ZH, GF, LX, YZ, RL, QL, JW, GY, CH and ZS contribute to the acquisition, analysis, or interpretation of data for the work. LH, XL, XW and ZH prepare the tissue samples. QL, JW and GY help in the experiments. All authors finally approved the manuscript version to be published. QZ is the guarantor of the article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethical Committee of Xuzhou Clinical College of Xuzhou Medical University and conducted in accordance with ethical standards.
Informed consent
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, J., Liang, Q., Wang, G. et al. Targeted delivery of nuclear targeting probe for bladder cancer using cyclic pentapeptide c(RGDfK) and acridine orange. Clin Transl Oncol 25, 375–383 (2023). https://doi.org/10.1007/s12094-022-02938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02938-0