Abstract
Background
For clinically low-risk stage III colorectal cancer, the decision on cycles of adjuvant chemotherapy after surgery is disputed. The present study investigates the use of additional biomarkers of ploidy and stroma-ratio(PS) to stratify patients with low-risk stage III colorectal cancer, providing a basis for individualized treatment in the future.
Methods
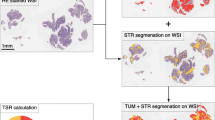
This study retrospectively enrolled 198 patients with clinical-low-risk stage III colorectal cancer (T1-3N1M0) and analyzed the DNA ploidy and stroma ratio of FFPE tumor tissues. The patients were divided into PS-low-risk group (Diploidy or Low-stroma) and PS-high-risk group (Non-diploid and High-stroma). For survival analyses, Kaplan–Meier and Cox regression models were used.
Results
The results showed that the 5-year DFS of the PS-high-risk group was significantly lower than that in the PS-low-risk group (78.6 vs. 91.2%, HR = 2.606 [95% CI: 1.011–6.717], P = 0.039). Besides, in the PS-low-risk group, the 5 year OS (98.2 vs. 86.7%, P = 0.022; HR = 5.762 [95% CI: 1.281–25.920]) and DFS (95.6, vs 79.9%, P = 0.019; HR = 3.7 [95% CI: 1.24–11.04]) of patients received adjuvant chemotherapy for > 3 months were significantly higher than those received adjuvant chemotherapy for < 3 months. We also found that the PS could stratify the prognosis of patients with dMMR tumors. The 5-year OS (96.3 vs 71.4%, P = 0.037) and DFS (92.6 vs 57.1%, P = 0.015) were higher in the PS-low-risk dMMR patients than those in the PS-high-risk dMMR patients.
Conclusion
In this study, we found that PS can predict the prognosis of patients with stage III low-risk CRC. Besides, it may guide the decision on postoperative adjuvant chemotherapy.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551.
Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. https://doi.org/10.3322/caac.21338.
Benson AB, et al. NCCN Guidelines Insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359–69. https://doi.org/10.6004/jnccn.2018.0021.
Des Guetz G, Uzzan B, Morere JF, Perret G, Nicolas P. Duration of adjuvant chemotherapy for patients with non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2010. https://doi.org/10.1002/14651858.CD007046.pub2.
Grothey A, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177–88. https://doi.org/10.1056/NEJMoa1713709.
Lieu C, et al. Duration of oxaliplatin-containing adjuvant therapy for stage III colon cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1436–47. https://doi.org/10.1200/JCO.19.00281.
Danielsen HE, Pradhan M, Novelli M. Revisiting tumour aneuploidy - the place of ploidy assessment in the molecular era. Nat Rev Clin Oncol. 2016;13:291–304. https://doi.org/10.1038/nrclinonc.2015.208.
Mesker WE, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387–98. https://doi.org/10.1155/2007/175276.
Wang K, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol. 2012;7:1457–61. https://doi.org/10.1097/JTO.0b013e318260dfe8.
Zhang XL, et al. The tumor-stroma ratio is an independent predictor for survival in nasopharyngeal cancer. Oncol Res Treat. 2014;37:480–4. https://doi.org/10.1159/000365165.
Chen Y, Zhang L, Liu W, Liu X. Prognostic significance of the tumor-stroma ratio in epithelial ovarian cancer. Biomed Res Int. 2015;2015: 589301. https://doi.org/10.1155/2015/589301.
Yang L, et al. Prognostic value of nucleotyping, DNA ploidy and stroma in high-risk stage II colon cancer. Br J Cancer. 2020;123:973–81. https://doi.org/10.1038/s41416-020-0974-8.
Danielsen HE, et al. Prognostic markers for colorectal cancer: estimating ploidy and stroma. Ann Oncol. 2018;29:616–23. https://doi.org/10.1093/annonc/mdx794.
Van Loo P, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107:16910–5. https://doi.org/10.1073/pnas.1009843107.
Argiles G, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291–305. https://doi.org/10.1016/j.annonc.2020.06.022.
Pages F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–39. https://doi.org/10.1016/S0140-6736(18)30789-X.
Auslander N, Wolf YI, Koonin EV. Interplay between DNA damage repair and apoptosis shapes cancer evolution through aneuploidy and microsatellite instability. Nat Commun. 2020;11:1234. https://doi.org/10.1038/s41467-020-15094-2.
Hveem TS, et al. Prognostic impact of genomic instability in colorectal cancer. Br J Cancer. 2014;110:2159–64. https://doi.org/10.1038/bjc.2014.133.
Carvalho B, et al. Concurrent hypermethylation of gene promoters is associated with a MSI-H phenotype and diploidy in gastric carcinomas. Eur J Cancer. 2003;39:1222–7. https://doi.org/10.1016/s0959-8049(03)00177-1.
Mouradov D, et al. Survival in stage II/III colorectal cancer is independently predicted by chromosomal and microsatellite instability, but not by specific driver mutations. Am J Gastroenterol. 2013;108:1785–93. https://doi.org/10.1038/ajg.2013.292.
Sinicrope FA, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131:729–37. https://doi.org/10.1053/j.gastro.2006.06.005.
Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–62. https://doi.org/10.1038/nrclinonc.2009.237.
Cohen R, et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: an ACCENT pooled analysis of 12 adjuvant trials. J Clin Oncol. 2021;39:642–51. https://doi.org/10.1200/JCO.20.01600.
Sargent DJ, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26. https://doi.org/10.1200/JCO.2009.27.1825.
Roth AD, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 2010;28:466–74. https://doi.org/10.1200/JCO.2009.23.3452.
Acknowledgements
We deeply appreciate the help from all of our colleagues in the Department of Colorectal Surgery at Sun Yat-sen University Cancer Center who were involved in performing the treatments for the current study. Meanwhile, we wish to thank the timely help given by Mao Lijun and Wang Fei in article writing and basic experiment.
Funding
This work was supported by grants from the National Natural Science Foundation of China [grant numbers: 81871971, 82073159, 81772595]; Sun Yat-sen University Clinical Research 5010 Program [grant number: 2014013].
Author information
Authors and Affiliations
Contributions
YL, LL, ZP and PD: contributed to the conception of the study; BX, QS, YX, WM, JY and ZH: collected data; WJ, LK, JT: and KH: contributed significantly to analysis and manuscript preparation; ZH, CZ, CZ and LZ: designed table and figure; YL and LL: performed the data analyses and wrote the manuscript; ZP and PD: helped perform the analysis with constructive discussions and contributed to critical revision; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest or financial ties to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Research Ethics Committee of Sun Yat-sen University Cancer Center (approval number: B2019-109).
Inform consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liao, L., Kong, L. et al. DNA ploidy and stroma predicted the risk of recurrence in low-risk stage III colorectal cancer. Clin Transl Oncol 25, 218–225 (2023). https://doi.org/10.1007/s12094-022-02930-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02930-8