Abstract
Purpose
This study was designed to explore the role of COPZ1 in breast cancer as well as discuss its specific reaction mechanism.
Methods
With the help of RT-qPCR and western blot, the expression of BMI1 and COPZ1 were measured. Then, the proliferation, colony formation and apoptosis were evaluated by CCK-8, colony formation and TUNEL assays, separately. Luciferase reporter assay and ChIP were applied to assess the relative activity of COPZ1 promoter as well as its binding with BMI1. Moreover, western blot was utilized to measure the expression of proliferation-, apoptosis- and autophagy-related proteins.
Results
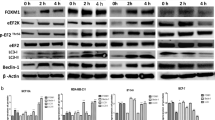
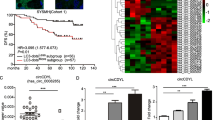
According to GEPIA2 database, COPZ1 was upregulated in breast cancer tissues and was associated with the poor prognosis (P = 0.03). Results obtained from RT-qPCR and western blot verified that COPZ1 expression was greatly increased at both mRNA and protein levels in breast cancer cells as compared to control cells (P < 0.05 or P < 0.001). COPZ1 knockdown inhibited the proliferation, induced the autophagy and promoted the apoptosis of breast cancer cells. HumanTFDB predicted the binding sites of BMI1 and COPZ1. The increased relative luciferase activity of COPZ1 promoter following BMI1 overexpression (P < 0.001) and the binding of BMI1 with COPZ1 promoter indicated that BMI1 could activate COPZ1. Further experiments suggested that the effects of COPZ1 knockdown on the proliferation, apoptosis and autophagy of breast cancer cells were reversed by BMI1 overexpression, implying that BMI1 promoted the proliferation and repressed the autophagy of breast cancer cells via activating COPZ1.
Conclusions
To sum up, BMI1 exhibited promotive effects on the malignant progression of breast cancer through the activation of COPZ1. These findings might offer a preliminary theoretical basis for COPZ1 participation in autophagy in breast cancer cells.






Similar content being viewed by others
References
Najafi S, Sadeghi M, Abasvandi F, et al. Prognostic factors influencing prognosis in early breast cancer patients. Prz Menopauzalny. 2019;18(2):82–8.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70.
Torti SV, Torti FM. Cellular iron metabolism in prognosis and therapy of breast cancer. Crit Rev Oncog. 2013;18(5):435–48.
Mayor S. Screening for early breast cancer reduces invasive cancer, study finds. BMJ. 2015;351: h6576.
Majeed W, Aslam B, Javed I, et al. Breast cancer: major risk factors and recent developments in treatment. Asian Pac J Cancer Prev. 2014;15(8):3353–8.
Beck R, Rawet M, Wieland FT, et al. The COPI system: molecular mechanisms and function. FEBS Lett. 2009;583(17):2701–9.
Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 2009;185(2):305–21.
Beller M, Sztalryd C, Southall N, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6(11): e292.
Shtutman M, Baig M, Levina E, et al. Tumor-specific silencing of COPZ2 gene encoding coatomer protein complex subunit zeta 2 renders tumor cells dependent on its paralogous gene COPZ1. Proc Natl Acad Sci USA. 2011;108(30):12449–54.
Huang X, Cho S, Spangrude GJ. Hematopoietic stem cells: generation and self-renewal. Cell Death Differ. 2007;14(11):1851–9.
Bhattacharya R, Mustafi SB, Street M, et al. Bmi-1: at the crossroads of physiological and pathological biology. Genes Dis. 2015;2(3):225–39.
Wang MC, Li CL, Cui J, et al. BMI-1, a promising therapeutic target for human cancer. Oncol Lett. 2015;10(2):583–8.
Ren H, Du P, Ge Z, et al. TWIST1 and BMI1 in cancer metastasis and chemoresistance. J Cancer. 2016;7(9):1074–80.
Chang J, Hu X, Nan J, et al. HOXD9induced SCNN1A upregulation promotes pancreatic cancer cell proliferation, migration and predicts prognosis by regulating epithelialmesenchymal transformation. Mol Med Rep. 2021. https://doi.org/10.3892/mmr.2021.12459.
Jurikova M, Danihel L, Polak S, et al. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016;118(5):544–52.
Kaczanowski S. Apoptosis: its origin, history, maintenance and the medical implications for cancer and aging. Phys Biol. 2016;13(3): 031001.
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34(7):856–80.
Zhang Y, Kong Y, Ma Y, et al. Loss of COPZ1 induces NCOA4 mediated autophagy and ferroptosis in glioblastoma cell lines. Oncogene. 2021;40(8):1425–39.
Di Marco T, Bianchi F, Sfondrini L, et al. COPZ1 depletion in thyroid tumor cells triggers type I IFN response and immunogenic cell death. Cancer Lett. 2020;476:106–19.
Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66(12):6225–32.
Dey A, Mustafi SB, Saha S, et al. Inhibition of BMI1 induces autophagy-mediated necroptosis. Autophagy. 2016;12(4):659–70.
Gergely JE, Dorsey AE, Dimri GP, et al. Timosaponin A-III inhibits oncogenic phenotype via regulation of PcG protein BMI1 in breast cancer cells. Mol Carcinog. 2018;57(7):831–41.
Funding
This project is funded by Fund for Distinguished Young Scholars of 900 Hospital (No. 2021JQ08) and Starup Fund for Scientific Research, Fujian Medical University (No. 2020QH1250).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, S., Li, H., Chen, S. et al. BMI1 promotes the proliferation and inhibits autophagy of breast cancer cells by activating COPZ1. Clin Transl Oncol 24, 2166–2174 (2022). https://doi.org/10.1007/s12094-022-02869-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02869-w