Abstract
Purpose
To assess the methodological quality of all relevant and recent European clinical practice guidelines (CPGs) for advanced oesophageal and gastric cancers, and to synthesise their recommendations on the use of chemotherapy.
Methods
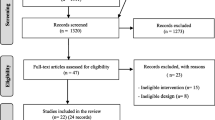
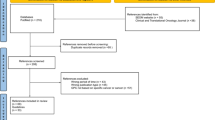
We searched PubMed, EMBASE, guidelines repositories, and other sources from 2010 onwards. We appraised quality using AGREE-II and AGREE-REX.
Results
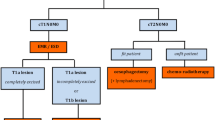
11 CPGs were included (five high, five low, and one moderate quality). Most guidelines showed deficiencies in the domain “applicability”, with only three scoring above 60%. Nine did not report having sought the views and preferences of the target population. The lowest scores for AGREE-REX were item Values and Preferences of Target Users (1.6; SD 1.3), and item Values and Preferences of Policy/Decision-Makers (1.8; SD 1.7). The domain Clinical Applicability got the highest score and the domain Implementability got the lowest.
Conclusions
An urgent area of research is how to develop credible and implementable recommendations on the clinical use of CT for advanced oesophageal and gastric cancer. Systematic review registration: PROSPERO (CRD42021236753).


Similar content being viewed by others
References
Bettio M, Carvalho RN, Dimitrova N, Dyba T. Measuring the cancer burden in Europe: The European Cancer Information System (ECIS). Ann Oncol. 2019;30:v675.
Hofmarcher T, Lindgren P. The cost of cancers of the digestive system in Europe. https://digestivecancers.eu/wp-content/uploads/2020/10/IHE_DiCE_HealthEcoStudy_2020.pdf. Accessed 9 Aug 2021.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–606.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62.
SEER Cancer Statistics Review, 1975–2018. https://seer.cancer.gov/csr/1975_2018/index.html. Accessed 9 Aug 2021.
Gavin AT, Francisci S, Foschi R, Donnelly DW, Lemmens V, Brenner H, Anderson LA, EUROCARE-4 Working Group. Oesophageal cancer survival in Europe: a EUROCARE-4 study. Cancer Epidemiol. 2012;36:505–12.
Schleicher SM, Bach PB, Matsoukas K, Korenstein D. Medication overuse in oncology: current trends and future implications for patients and society. Lancet Oncol. 2018;19:e200–8.
Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, Nagpal S, Saini V, Srivastava D, Chalmers K, et al. Evidence for overuse of medical services around the world. Lancet. 2017;390:156–68.
Santero M, Pérez-Bracchiglione J, Acosta-Dighero R, Meade AG, Antequera A, Auladell-Rispau A, Quintana MJ, Requeijo C, Rodríguez-Grijalva G, Salas-Gama K, et al. Efficacy of systemic oncological treatments in patients with advanced esophageal or gastric cancers at high risk of dying in the middle and short term: an overview of systematic reviews. BMC Cancer. 2021;21:712.
Alonso-Coello P, Irfan A, Sola I, Gich I, Delgado-Noguera M, Rigau D, Tort S, Bonfill X, Burgers J, Schunemann H. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. BMJ Qual Saf. 2010;19:e58–e58. https://doi.org/10.1136/qshc.2010.042077.
Johnston A, Kelly SE, Hsieh S-C, Skidmore B, Wells GA. Systematic reviews of clinical practice guidelines: a methodological guide. J Clin Epidemiol. 2019;108:64–76.
Brouwers MC, Kerkvliet K, Spithoff K. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152.
Iturrioz RR, Gutiérrez-Ibarluzea I, Batarrita JA, Puerto MAN, Domínguez AR, León IM, de la Blanca EBP. Valoración de escalas y criterios para la evaluación de guías de práctica clínica. Rev Esp Salud Publica. 2004. https://doi.org/10.1590/s1135-57272004000400004.
Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10.
Dans AL, Dans LF. Appraising a tool for guideline appraisal (the AGREE II instrument). J Clin Epidemiol. 2010;63:1281–2.
Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D. A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17:235–42.
Brouwers MC, Spithoff K, Kerkvliet K, Alonso-Coello P, Burgers J, Cluzeau F, Férvers B, Graham I, Grimshaw J, Hanna S, et al. Development and validation of a tool to assess the quality of clinical practice guideline recommendations. JAMA Netw Open. 2020;3: e205535.
Yepes-Nuñez JJ, Urrútia G, Romero-García M, Alonso-Fernández S. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Revista Española de Cardiología. 2021. https://www.sciencedirect.com/science/article/pii/S0300893221002748.
Santero M, Melendi SE, Gonzalez L, Acosta-Dighero R, Solà I, Meade A-G, Quintana MJ, Urrutia G, Bonfill X. Paper 1: Clinical practice guidelines on the use of chemotherapy for nonintestinal digestive advanced cancer in Europe: a critical appraisal using the AGREE II instrument. 2021. https://osf.io/dq3ay/. Accessed 26 Jul 2021.
Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E. Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice G. Washington: Clinical Practice Guidelines We Can Trust. National Academies Press (US) Copyright; 2011.
Veritas Health Innovation. Covidence systematic review software. https://www.covidence.org/. Accessed 9 Aug 2021.
Makarski J, Brouwers MC. The AGREE Enterprise: a decade of advancing clinical practice guidelines. Implement Sci. 2014. https://doi.org/10.1186/s13012-014-0103-2.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8.
McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. https://doi.org/10.1037/1082-989x.1.1.30.
R Core Team. R: A language and environment for statistical computing. 2021. www.r-project.org.
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–7.
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D, ESMO Guidelines Working Group. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):51–6.
Stahl M, Budach W, Meyer H-J, Cervantes A, ESMO Guidelines Working Group. Esophageal cancer: Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v46–9.
Committee NCE, Others. Diagnosis, staging and treatment of patients with oesophageal or oesophagogastric junction cancer: National Clinical Guideline No. 19: Summary. https://www.lenus.ie/bitstream/handle/10147/626876/3f3d0e24578b43279e31ffac30193cb7.pdf?sequence=1.
Martin-Richard M, Díaz Beveridge R, Arrazubi V, Alsina M, Galan Guzmán M, Custodio AB, Gómez C, Muñoz FL, Pazo R, Rivera F. SEOM Clinical Guideline for the diagnosis and treatment of esophageal cancer (2016). Clin Transl Oncol. 2016;18:1179–86.
Gallego J, Cervantes A, Pericay C, Isla D. SEOM clinical guidelines for the treatment of oesophageal cancer. Clin Transl Oncol. 2011;13:520–4.
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–49.
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, European Society for Medical Oncology (ESMO), European Society of Surgical Oncology (ESSO), European Society of Radiotherapy and Oncology (ESTRO) . Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi57-63.
Okines A, Verheij M, Allum W, Cunningham D, Cervantes A, ESMO Guidelines Working Group. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v50–4.
Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, Fares N, Granger V, Lefort C, Gagniere J, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 2018;50:768–79.
De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei MA, Pacelli F, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastr Cancer. 2017;20:20–30.
Alsina Maqueda M, Boladeras Inglada AM, Bugès Sanchez C, Calvo-Campos M, Canals-Subirats E, Caro-Gallarín M, Creus Costas G, Fort-Casamartina E, Galán Guzmán M, Gilabert-Sotoca M, et al. ICO-ICS Praxi per al tractament mèdic i amb irradiació de càncer gàstric i d’unió esofagogàstrica. 2019. http://hdl.handle.net/11351/6061. Accessed 17 Aug 2021.
Martín-Richard M, Carmona-Bayonas A, Custodio AB, Gallego J, Jiménez-Fonseca P, Reina JJ, Richart P, Rivera F, Alsina M, Sastre J. SEOM clinical guideline for the diagnosis and treatment of gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJA) (2019). Clin Transl Oncol. 2020;22:236–44.
Rivera F, Grávalos C, García-Carbonero R, SEOM (Spanish Society of Clinical Oncology). SEOM clinical guidelines for the diagnosis and treatment of gastric adenocarcinoma. Clin Transl Oncol. 2012;14:528–35.
Lerut T, Stordeur S, Verleye L. Update of the national guideline on upper gastrointestinal cancer-appendix. Good Clinical Practice (GCP). Brussels: Belgian Health Care Knowledge Centre (KCE). 2012. KCE Report 179S. D/2012/10.273/35. https://kce.fgov.be/sites/default/files. 2014.
Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R, Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–72.
National Institute for Health and Care Excellence (Great Britain). Oesophago-gastric cancer: assessment and management in adults. 2018.
Bauer K, Schroeder M, Porzsolt F, Henne-Bruns D. Comparison of international guidelines on the accompanying therapy for advanced gastric cancer: reasons for the differences. J Gastr Cancer. 2015;15:10–8.
Gärtner FR, Portielje JE, Langendam M, Hairwassers D, Agoritsas T, Gijsen B, Liefers G-J, Pieterse AH, Stiggelbout AM. Role of patient preferences in clinical practice guidelines: a multiple methods study using guidelines from oncology as a case. BMJ Open. 2019;9: e032483.
Homepage—Digestive Cancer Europe [Internet]. 2020. https://digestivecancers.eu/. Accessed 24 Aug 2021.
Braga S. Why do our patients get chemotherapy until the end of life? Ann Oncol. 2011;22:2345–8.
Florez ID, Brouwers MC, Kerkvliet K, Spithoff K, Alonso-Coello P, Burgers J, Cluzeau F, Férvers B, Graham I, Grimshaw J, et al. Assessment of the quality of recommendations from 161 clinical practice guidelines using the Appraisal of Guidelines for Research and Evaluation-Recommendations Excellence (AGREE-REX) instrument shows there is room for improvement. Implement Sci. 2020;15:1–8.
Selva A, Sanabria AJ, Pequeño S, Zhang Y, Solà I, Pardo-Hernandez H, Selva C, Schünemann H, Alonso-Coello P. Incorporating patients’ views in guideline development: a systematic review of guidance documents. J Clin Epidemiol. 2017;88:102–12.
Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, Lampert U, Eikermann M. Guideline appraisal with AGREE II: systematic review of the current evidence on how users handle the 2 overall assessments. PLoS ONE. 2017;12: e0174831.
Acknowledgements
Marilina Santero is a doctoral candidate for the Ph.D. in Methodology of Biomedical Research and Public Health. Universitat Autònoma de Barcelona, Barcelona, Spain.
Funding
Funding was provided by Instituto de Salud Carlos III (Grant nos. PFIS FI19/00335, PI18/00034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
This is a systematic review article that doesn’t need informed consent or any ethical approvals in its current form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santero, M., Meade, A., Acosta-Dighero, R. et al. European clinical practice guidelines on the use of chemotherapy for advanced oesophageal and gastric cancers: a critical review using the AGREE II and the AGREE-REX instruments. Clin Transl Oncol 24, 1588–1604 (2022). https://doi.org/10.1007/s12094-022-02807-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02807-w