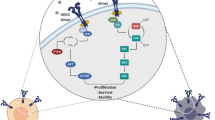
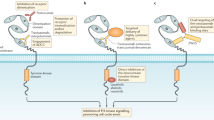
Abstract
Gastric cancer is one of the most common malignancy worldwide with a prognosis less than 1 year in unresectable or metastatic disease. HER2 expression is the main biomarker to lead the addition of trastuzumab to first line systemic chemotherapy improving the overall survival in advanced HER2-positivegastric adenocarcinoma. The inevitable development of resistance to trastuzumab remains a great problem inasmuch several treatment strategies that have proven effective in breast cancer failed to show clinical benefit in advanced gastric cancer. In this review, we summarize the available data on the mechanisms underlying primary and secondary resistance toHER2-targeted therapy and current challenges in the treatment of HER2-positive advanced gastric cancer refractory to trastuzumab. Further, we describe the prognostic value of new non-invasive screening techniques, the current development of novel agents such us HER2 antibody–drug conjugates and bispecific antibodies, and the strategies with antitumor activity on going.


Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2019 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020. https://doi.org/10.3322/caac.21660.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–9. https://doi.org/10.1093/annonc/mdn169.
Rugge M, Fassan M, Graham DY. Epidemiology of gastric cancer. Gastric Cancer Principles Pract. 2015. https://doi.org/10.1007/978-3-319-15826-6_2.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–64. https://doi.org/10.1016/S0140-6736(16)30354-3.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–9. https://doi.org/10.1200/JCO.2005.05.0245.
Fontana E, Smyth EC. Novel targets in the treatment of advanced gastric cancer: a perspective review. Therap Adv Med Oncol. 2016;8(2):113–25. https://doi.org/10.1177/1758834015616935.
Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–49. https://doi.org/10.1093/annonc/mdw350.
Zaanan A, Bouché O, Benhaim L, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 2018;50(8):768–79. https://doi.org/10.1016/j.dld.2018.04.025.
Petrioli R, Francini E, Roviello F, et al. Sequential treatment with epirubicin, oxaliplatin and 5FU (EOF) followed by docetaxel, oxaliplatin and 5FU (DOF) in patients with advanced gastric or gastroesophageal cancer: a single-institution experience. Cancer Chemother Pharmacol. 2015;75(5):941–7. https://doi.org/10.1007/s00280-015-2715-x.
Bass AJ, Thorsson V, Shmulevich I, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. https://doi.org/10.1038/nature13480.
Riese DJ, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays. 1998;20(1):41–8. https://doi.org/10.1002/(SICI)1521-1878(199801)20:1%3c41::AID-BIES7%3e3.0.CO;2-V.
Van Der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. https://doi.org/10.1146/annurev.cb.10.110194.001343.
Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:1–9. https://doi.org/10.1155/2014/852748.
Padhy LC, Shih C, Cowing D, Finkelstein R, Weinberg RA. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982;28(4):865–71. https://doi.org/10.1016/0092-8674(82)90065-4.
Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312(5994):513–6. https://doi.org/10.1038/312513a0.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–87. https://doi.org/10.1038/sj.onc.1210477.
Olayioye MA. Intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3(6):385–9. https://doi.org/10.1186/bcr327.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. https://doi.org/10.1038/nrm1962.
Nahta R, Yuan LXH, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Can Res. 2005;65(23):11118–28. https://doi.org/10.1158/0008-5472.CAN-04-3841.
Lee JW, Soung YH, Seo SH, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res. 2006;12(1):57–61. https://doi.org/10.1158/1078-0432.CCR-05-0976.
Hollywood DP, Hurst HC. A novel transcription factor, OB2-1, is required for overexpression of the proto-oncogene c-erbB-2 in mammary tumour lines. EMBO J. 1993;12(6):2369–75. https://doi.org/10.1002/j.1460-2075.1993.tb05891.x.
Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001. https://doi.org/10.1093/annonc/12.suppl_1.S9.
Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22(43):6570–8. https://doi.org/10.1038/sj.onc.1206779.
Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353(16):1652–4. https://doi.org/10.1056/nejmp058197.
Meza-Junco J, Au HJ, Sawyer MB. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. Cancer Manag Res. 2011;3(1):57–64. https://doi.org/10.2147/CMR.S12698.
Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50. https://doi.org/10.1038/modpathol.2011.198.
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59(5):832–40. https://doi.org/10.1111/j.1365-2559.2011.04017.x.
Koro K, Swanson PE, Yeh MM. HER2 testing in gastric and gastroesophageal adenocarcinoma. AJSP Rev Rep
Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer. 2012;3(1):137–44. https://doi.org/10.7150/jca.4090.
Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18(11):2201–9. https://doi.org/10.1200/JCO.2000.18.11.2201.
Yonemura Y, Tanaka M, Sasaki T, Fushida S, Kimura H, Ohoyama S. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Can Res. 1991;51(3):1034–8.
Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16(2):273–8. https://doi.org/10.1093/annonc/mdi064.
Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45. https://doi.org/10.1200/JCO.2006.09.2775.
Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312(5994):545–8. https://doi.org/10.1038/312545a0.
Brufsky A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: From early scientific development to foundation of care. Am J Clin Oncol Cancer Clin Trials. 2010;33(2):186–95. https://doi.org/10.1097/COC.0b013e318191bfb0.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. https://doi.org/10.1016/S0140-6736(10)61121-X.
Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. https://doi.org/10.1111/j.1365-2559.2008.03028.x.
Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110(5):1163–8. https://doi.org/10.1038/bjc.2014.18.
Ryu MH, Yoo C, Kim JG, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51(4):482–8. https://doi.org/10.1016/j.ejca.2014.12.015.
Gong J, Liu T, Fan Q, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): A multicenter, phase II trial. BMC Cancer. 2016. https://doi.org/10.1186/s12885-016-2092-9.
Takahari D, Chin K, Ishizuka N, et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22(6):1238–46. https://doi.org/10.1007/s10120-019-00973-5.
Shah MA, Xu RH, Bang YJ, et al. HELOISE: Phase IIIb randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2–positive metastatic gastric or gast. J Clin Oncol. 2017. https://doi.org/10.1200/JCO.2016.71.6852.
ter Veer E, Creemers A, de Waal L, van Oijen MGH, van Laarhoven HWM. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: a meta-analysis. Int J Cancer. 2018;143(2):438–48. https://doi.org/10.1002/ijc.31325.
Chaganty BKR, Lu Y, Qiu S, Somanchi SS, Lee DA, Fan Z. Trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules through engagement of natural killer cells and stimulation of IFNγ secretion. OncoImmunology. 2016. https://doi.org/10.1080/2162402X.2015.1100790.
Janjigian YY, Chou JF, Simmons M, et al. First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma (mEGA). J Clin Oncol. 2019;37(4):62–62. https://doi.org/10.1200/jco.2019.37.4_suppl.62.
Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24(41):6213–21. https://doi.org/10.1038/sj.onc.1208774.
Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—A randomized phase III trial. J Clin Oncol. 2016;34(5):443–51. https://doi.org/10.1200/JCO.2015.62.6598.
Harbeck N, Beckmann MW, Rody A, et al. HER2 dimerization inhibitor pertuzumab—Mode of action and clinical data in breast cancer. Breast Care. 2013;8(1):49–55. https://doi.org/10.1159/000346837.
von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–31. https://doi.org/10.1056/nejmoa1703643.
Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. https://doi.org/10.1056/nejmoa1113216.
Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84. https://doi.org/10.1016/S1470-2045(18)30481-9.
Satoh T, Doi T, Ohtsu A, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—A randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49. https://doi.org/10.1200/JCO.2013.53.6136.
Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53. https://doi.org/10.1016/S1470-2045(17)30111-0.
Von Minckwitz G, Du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27(12):1999–2006. https://doi.org/10.1200/JCO.2008.19.6618.
Li Q, Jiang H, Li H, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7(31):50656–65. https://doi.org/10.18632/oncotarget.10456.
Palle J, Tougeron D, Pozet A, et al. Trastuzumab beyond progression in patients with HER2-positive advanced gastric adenocarcinoma: a multicenter AGEO study. Oncotarget. 2017;8(60):101383–93. https://doi.org/10.18632/oncotarget.20711.
Narita Y, Kadowaki S, Masuishi T, et al. Correlation between human epidermal growth factor receptor 2 expression level and efficacy of trastuzumab beyond progression in metastatic gastric cancer. Oncol Lett. 2017;14(2):2545–51. https://doi.org/10.3892/ol.2017.6409.
Horita Y, Nishino M, Sugimoto S, et al. Phase II clinical trial of second-line weekly paclitaxel plus trastuzumab for patients with HER2-positive metastatic gastric cancer. Anticancer Drugs. 2019;30(1):98–104. https://doi.org/10.1097/CAD.0000000000000691.
Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). J Clin Oncol. 2018;36(15):4011–4011. https://doi.org/10.1200/jco.2018.36.15_suppl.4011.
Kijima T, Arigami T, Uenosono Y, et al. Comparison of HER2 Status before and after Trastuzumab-based chemotherapy in patients with advanced gastric cancer. Anticancer Res. 2020;40(1):75–80. https://doi.org/10.21873/anticanres.13927.
Fornaro L, Vivaldi C, Parnofiello A, et al. Validated clinico-pathologic nomogram in the prediction of HER2 status in gastro-oesophageal cancer. Br J Cancer. 2019;120(5):522–6. https://doi.org/10.1038/s41416-019-0399-4.
Lee HE, Park KU, Yoo SB, et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49(6):1448–57. https://doi.org/10.1016/j.ejca.2012.10.018.
Kim J, Fox C, Peng S, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Investig. 2014;124(12):5145–58. https://doi.org/10.1172/JCI75200.
Deguchi Y, Okabe H, Oshima N, et al. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer. 2017;20(3):416–27. https://doi.org/10.1007/s10120-016-0627-z.
Kim C, Lee CK, Chon HJ, et al. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget. 2017;8(69):113494–501. https://doi.org/10.18632/oncotarget.23054.
Huang LT, Ma JT, Zhang SL, et al. Durable clinical response to pyrotinib after resistance to prior anti-HER2 therapy for HER2-positive advanced gastric cancer: a case report. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.01453.
Minuti G, Cappuzzo F, Duchnowska R, et al. Increased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2-positive metastatic breast cancer. Br J Cancer. 2012;107(5):793–9. https://doi.org/10.1038/bjc.2012.335.
Takahashi N, Furuta K, Taniguchi H, et al. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget. 2016;7(4):4925–38. https://doi.org/10.18632/oncotarget.6753.
Kwak EL, Ahronian LG, Siravegna G, et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-Amplified esophagogastric cancer. Cancer Discov. 2015;5(12):1271–81. https://doi.org/10.1158/2159-8290.CD-15-0748.
Pietrantonio F, Fuca G, Morano F, et al. Biomarkers of primary resistance to trastuzumab in HER2-positive metastatic gastric cancer patients: the AMNESIA case-control study. Clin Cancer Res. 2018;24(5):1082–9. https://doi.org/10.1158/1078-0432.CCR-17-2781.
Vernieri C, Milano M, Brambilla M, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol. 2019;139:53–66. https://doi.org/10.1016/j.critrevonc.2019.05.001.
Palle J, Rochand A, Pernot S, Gallois C, Taïeb J, Zaanan A. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs. 2020;80(4):401–15. https://doi.org/10.1007/s40265-020-01272-5.
Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104(9):1372–6. https://doi.org/10.1038/bjc.2011.121.
Zang ZJ, Ong CK, Cutcutache I, et al. Genetic and structural variation in the gastric cancer kinome revealed through targeted deep sequencing. Can Res. 2011;71(1):29–39. https://doi.org/10.1158/0008-5472.CAN-10-1749.
Ucar DA, Kurenova E, Garrett TJ, et al. Disruption of the protein interaction between FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle. 2012;11(17):3250–9. https://doi.org/10.4161/cc.21611.
Arienti C, Zanoni M, Pignatta S, et al. Preclinical evidence of multiple mechanisms underlying trastuzumab resistance in gastric cancer. Oncotarget. 2016;7(14):18424–39. https://doi.org/10.18632/oncotarget.7575.
Gambardella V, Gimeno-Valiente F, Tarazona N, et al. Nrf2 through RPs6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res. 2019;25(5):1639–49. https://doi.org/10.1158/1078-0432.CCR-18-2421.
Piro G, Carbone C, Cataldo I, et al. An FGFR3 autocrine loop sustains acquired resistance to trastuzumab in gastric cancer patients. Clin Cancer Res. 2016;22(24):6164–75. https://doi.org/10.1158/1078-0432.CCR-16-0178.
Mao L, Sun A, Wu JZ, Tang J. Involvement of microRNAs in HER2 signaling and trastuzumab treatment. Tumor Biol. 2016;37(12):15437–46. https://doi.org/10.1007/s13277-016-5405-3.
Sui M, Jiao A, Zhai H, et al. Upregulation of miR-125b is associated with poor prognosis and trastuzumab resistance in HER2-positive gastric cancer. Exp Ther Med. 2017;14(1):657–63. https://doi.org/10.3892/etm.2017.4548.
Kim HP, Han SW, Song SH, et al. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33(25):3334–41. https://doi.org/10.1038/onc.2013.285.
Shi J, Wang Y, Zeng L, et al. Disrupting the interaction of BRD4 with diacetylated twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25(2):210–25. https://doi.org/10.1016/j.ccr.2014.01.028.
Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S. B7–H3 expression in gastric cancer: A novel molecular blood marker for detecting circulating tumor cells. Cancer Sci. 2011;102(5):1019–24. https://doi.org/10.1111/j.1349-7006.2011.01877.x.
Tsujiura M, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Otsuji E. Liquid biopsy of gastric cancer patients: circulating tumor cells and cell-free nucleic acids. World J Gastroenterol. 2014;20(12):3265–86. https://doi.org/10.3748/wjg.v20.i12.3265.
Liu Y, Ling Y, Qi Q, et al. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol. 2017;6(2):235–42. https://doi.org/10.3892/mco.2017.1125.
Wang S, Zheng G, Cheng B, et al. Circulating tumor cells (CTCs) detected by RT-PCR and its prognostic role in gastric cancer: a meta-analysis of published literature. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0099259.
Akca H, Demiray A, Yaren A, et al. Utility of serum DNA and pyrosequencing for the detection of EGFR mutations in non-small cell lung cancer. Cancer Genet. 2013;206(3):73–80. https://doi.org/10.1016/j.cancergen.2013.01.005.
Neumann MHD, Bender S, Krahn T, Schlange T. ctDNA and CTCs in liquid biopsy—current status and where we need to Progress. Comput Struct Biotechnol J. 2018;16:190–5. https://doi.org/10.1016/j.csbj.2018.05.002.
Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014. https://doi.org/10.1126/scitranslmed.3007094.
Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–91. https://doi.org/10.1158/2159-8290.CD-15-1483.
Sumbal S, Javed A, Afroze B, et al. Circulating tumor DNA in blood: Future genomic biomarkers for cancer detection. Exp Hematol. 2018;65:17–28. https://doi.org/10.1016/j.exphem.2018.06.003.
Kinugasa H, Nouso K, Tanaka T, et al. Droplet digital PCR measurement of HER2 in patients with gastric cancer. Br J Cancer. 2015;112(10):1652–5. https://doi.org/10.1038/bjc.2015.129.
Shoda K, Masuda K, Ichikawa D, et al. HER2 amplification detected in the circulating DNA of patients with gastric cancer: a retrospective pilot study. Gastric Cancer. 2015;18(4):698–710. https://doi.org/10.1007/s10120-014-0432-5.
Maron SB, Chase LM, Lomnicki S, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25(23):7098–112. https://doi.org/10.1158/1078-0432.CCR-19-1704.
Wang H, Li B, Liu Z, et al. HER2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur J Cancer. 2018;88:92–100. https://doi.org/10.1016/j.ejca.2017.10.032.
Riquet M, Rivera C, Gibault L, et al. Extension lymphatique du cancer du poumon: Une anatomie enchaînée dans des zones. Rev Pneumol Clin. 2014;70(1–2):16–25. https://doi.org/10.1016/j.pneumo.2013.07.001.
Mavroudis D. Circulating cancer cells. Ann Oncol. 2010. https://doi.org/10.1093/annonc/mdq378.
Tseng JY, Yang CY, Liang SC, Liu RS, Jiang JK, Lin CH. Dynamic changes in numbers and properties of circulating tumor cells and their potential applications. Cancers. 2014;6(4):2369–86. https://doi.org/10.3390/cancers6042369.
Nevisi F, Yaghmaie M, Pashaiefar H, et al. Correlation of HER2, MDM2, c-MYC, c-MET, and TP53 copy number alterations in circulating tumor cells with tissue in gastric cancer patients: A pilot study. Iran Biomed J. 2020;24(1):47–53. https://doi.org/10.29252/ibj.24.1.47.
Mishima Y, Matsusaka S, Chin K, et al. Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target Oncol. 2017;12(3):341–51. https://doi.org/10.1007/s11523-017-0493-6.
Peng Z, Liu Y, Li Y, et al. Serum HER2 extracellular domain as a potential alternative for tissue HER2 status in metastatic gastric cancer patients. Biomark Med. 2014;8(5):663–70. https://doi.org/10.2217/bmm.14.10.
Witzel I, Loibl S, Von Minckwitz G, et al. Predictive value of HER2 serum levels in patients treated with lapatinib or trastuzumab-a translational project in the neoadjuvant GeparQuinto trial. Br J Cancer. 2012;107(6):956–60. https://doi.org/10.1038/bjc.2012.353.
Lipton A, Leitzel K, Ali SM, et al. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117(21):5013–20. https://doi.org/10.1002/cncr.26101.
Janjigian YY, Viola-Villegas N, Holland JP, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and89Zr-trastuzumab PET. J Nucl Med. 2013;54(6):936–43. https://doi.org/10.2967/jnumed.112.110239.
O’Donoghue JA, Lewis JS, Pandit-Taskar N, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89 Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med. 2018;59(1):161–6. https://doi.org/10.2967/jnumed.117.194555.
Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108. https://doi.org/10.1158/1078-0432.CCR-15-2822.
Shi J, Li F, Yao X, et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 2018;37(22):3022–38. https://doi.org/10.1038/s41388-018-0204-5.
Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a)in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):827–36. https://doi.org/10.1016/S1470-2045(19)30088-9.
Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512–22. https://doi.org/10.1016/S1470-2045(17)30604-6.
Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30. https://doi.org/10.1056/nejmoa2004413.
Takegawa N, Tsurutani J, Kawakami H, et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int J Cancer. 2019;145(12):3414–24. https://doi.org/10.1002/ijc.32408.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. https://doi.org/10.1111/cas.12966.
Catenacci DVT, Kang YK, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–76. https://doi.org/10.1016/S1470-2045(20)30326-0.
Catenacci DVT, Lim KH, Uronis HE, et al. Antitumor activity of margetuximab (M) plus pembrolizumab (P) in patients (pts) with advanced HER2+ (IHC3+) gastric carcinoma (GC). J Clin Oncol. 2019;37(4):65. https://doi.org/10.1200/jco.2019.37.4_suppl.65.
Combination Margetuximab, INCMGA00012, MGD013, and Chemotherapy Phase 2 / 3 Trial in HER2+ Gastric / GEJ Cancer (MAHOGANY) - Cerca con Google.
ZW25 effective in HER2-positive cancers. Cancer Discov. 2019;9(1):8. https://doi.org/10.1158/2159-8290.CD-NB2018-162
Meric-Bernstam F, Beeram M, Mayordomo JI, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol. 2018;36(15):2500. https://doi.org/10.1200/jco.2018.36.15_suppl.2500.
O’Donovan N, Byrne AT, O’Connor AE, McGee S, Gallagher WM, Crown J. Synergistic interaction between trastuzumab and EGFR/HER-2 tyrosine kinase inhibitors in HER-2 positive breast cancer cells. Invest New Drugs. 2011;29(5):752–9. https://doi.org/10.1007/s10637-010-9415-5.
Sanchez-Vega F, Hechtman JF, Castel P, et al. Egfr and MET amplifications determine response to HER2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 2019;9–2:199–209. https://doi.org/10.1158/2159-8290.CD-18-0598.
Minkovsky N, Berezov A. BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008;9(12):1336–46.
Kim TY, Han HS, Lee KW, et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22(6):1206–14. https://doi.org/10.1007/s10120-019-00958-4.
Oh DY, Lee KW, Cho JY, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer. 2016;19(4):1095–103. https://doi.org/10.1007/s10120-015-0567-z.
Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol. 2019;133:25–32. https://doi.org/10.1016/j.critrevonc.2018.10.007.
Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33. https://doi.org/10.1016/S0140-6736(18)31257-1.
Tabernero J, Van Cutsem E, Bang Y-J, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(18):4007. https://doi.org/10.1200/jco.2019.37.18_suppl.lba4007.
Bang YJ, Yañez Ruiz E, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–60. https://doi.org/10.1093/annonc/mdy264.
Kiyozumi Y, Iwatsuki M, Yamashita K, Koga Y, Yoshida N, Baba H. Update on targeted therapy and immune therapy for gastric cancer, 2018. J Cancer Metastas Treat. 2018;4(6):31. https://doi.org/10.20517/2394-4722.2017.77.
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5.
Satoh T, Kang YK, Chao Y, et al. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23(1):143–53. https://doi.org/10.1007/s10120-019-00970-8.
Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with s-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (a. Ann Oncol. 2019;30(2):250–8. https://doi.org/10.1093/annonc/mdy540.
Janjigian YY, Adenis A, Aucoin J-S, et al. Checkmate 649: A randomized, multicenter, open-label, phase 3 study of nivolumab (Nivo) plus ipilimumab (Ipi) versus oxaliplatin plus fluoropyrimidine in patients (Pts) with previously untreated advanced or metastatic gastric (G) or gastroesophageal junct. J Clin Oncol. 2017;35(4):213. https://doi.org/10.1200/jco.2017.35.4_suppl.tps213.
Kelly RJ, Chung K, Gu Y, et al. Phase Ib/II study to evaluate the safety and antitumor activity of durvalumab (MEDI4736) and tremelimumab as monotherapy or in combination, in patients with recurrent or metastatic gastric/gastroesophageal junction adenocarcinoma. J ImmunoTherapy Cancer. 2015. https://doi.org/10.1186/2051-1426-3-s2-p157.
Kelly RJ, Lee J, Bang Y-J, et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. J Clin Oncol. 2018;36(15):4031–4031. https://doi.org/10.1200/jco.2018.36.15_suppl.4031.
Moehler M, Ryu MH, Dvorkin M, et al. Maintenance avelumab versus continuation of first-line chemotherapy in gastric cancer: JAVELIN Gastric 100 study design. Future Oncol. 2019;15(6):567–77. https://doi.org/10.2217/fon-2018-0668.
JapicCTI J. An Investigational Immuno-therapy Study to Assess the Safety, Tolerability and Effectiveness of Anti-LAG-3 With and Without Anti-PD-1 in the Treatment of Solid Tumors. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-183890. Published online 2018.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author declares any conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roviello, G., Catalano, M., Iannone, L.F. et al. Current status and future perspectives in HER2 positive advanced gastric cancer. Clin Transl Oncol 24, 981–996 (2022). https://doi.org/10.1007/s12094-021-02760-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02760-0