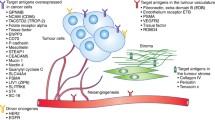
Abstract
An antibody–drug conjugate (ADC) is an advanced chemotherapeutic option with immense promises in treating many tumor. They are designed to selectively attack and kill neoplastic cells with minimal toxicity to normal tissues. ADCs are complex engineered immunoconjugates that comprise a monoclonal antibody for site-directed delivery and cytotoxic payload for targeted destruction of malignant cells. Therefore, it enables the reduction of off-target toxicities and enhances the therapeutic index of the drug. Hepatocellular carcinoma (HCC) is a solid tumor that shows high heterogeneity of molecular phenotypes and is considered the second most common cause of cancer-related death. Studies show enormous potential for ADCs targeting GPC3 and CD24 and other tumor-associated antigens in HCC with their high, selective expression and show potential outputs in preclinical evaluations. The review mainly highlights the preclinical evaluation of different antigen-targeted ADCs such as MetFab-DOX, Anti-c-Met IgG-OXA, Anti CD 24, ANC–HN-01, G7mab-DOX, hYP7-DCand hYP7-PC, Anti-CD147 ILs-DOX and AC133-vcMMAF against hepatocellular carcinoma and its future relevance.
Graphic abstract






Similar content being viewed by others
References
Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;28(10):171. https://doi.org/10.3389/fonc.2020.00171.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2.
World Health Organization.International Agency for Research on Cancer. 2021.https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf. Accessed Mar 2021.
Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M. Immunotherapy in hepatocellular carcinoma. Ann Hepatol. 2019;18(2):291–7. https://doi.org/10.1016/j.aohep.2019.04.003.
Wang SZ, Lee SD, Sarkar D, Lee HM, Khan A, Bhati C, et al. Immunological characterization of hepatocellular carcinoma. Hepatoma Res. 2021;7:6. https://doi.org/10.20517/2394-5079.2020.113.
Nishida N, Kudo M. Oncogenic signal and tumour microenvironment in hepatocellular carcinoma. Oncology. 2017;93(Suppl. 1):160–4. https://doi.org/10.1159/000481246.
Nishida N, Kudo M. Immunological microenvironment of hepatocellular carcinoma and its clinical implication. Oncology. 2017;92(Suppl. 1):40–9. https://doi.org/10.1159/000451015.
Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015;7(17):2080. https://doi.org/10.4254/wjh.v7.i17.2080.
Feun LG, Li YY, Wangpaichitr M, Wu CJ, Savaraj N. Immunotherapy for hepatocellular carcinoma: the force awakens in HCC? Hepatoma Res. 2017;3:43–51. https://doi.org/10.20517/2394-5079.2016.45.
Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62(6):1420–9. https://doi.org/10.1016/j.jhep.2015.02.038.
Chaoul N, Mancarella S, Lupo L, Giannelli G, Dituri F. Impaired anti-tumor T cell response in hepatocellular carcinoma. Cancers. 2020;12(3):627. https://doi.org/10.3390/cancers12030627.
Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer cell. 2006;10(2):99–111. https://doi.org/10.1016/j.ccr.2006.06.016.
Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64(6):2038–46. https://doi.org/10.1002/hep.28710.
Parayath N, Padmakumar S, Nair SV, Menon D, Amiji MM. Strategies for targeting cancer immunotherapy through modulation of the tumour microenvironment. Regen Eng Transl Med. 2020;6(1):29–49. https://doi.org/10.1007/s40883-019-00113-6.
Greten TF, Lai CW, Li G, Staveley-O’Carroll KF. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology. 2019;156(2):510–24. https://doi.org/10.1053/j.gastro.2018.09.051.
Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70(1):204–14. https://doi.org/10.1136/gutjnl-2020-321702.
Lu LC, Hsu CH, Hsu C, Cheng AL. Tumor heterogeneity in hepatocellular carcinoma: facing the challenges. Liver cancer. 2016;5(2):128–38. https://doi.org/10.1159/000367754.
Dahlgren D, Lennernäs H. Antibody-drug conjugates and targeted treatment strategies for hepatocellular carcinoma: a drug-delivery perspective. Molecules. 2020;25(12):2861. https://doi.org/10.3390/molecules25122861.
Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. https://doi.org/10.4103/jcar.JCar_9_16.
Kumari R, Sahu MK, Tripathy A, Uthansingh K, Behera M. Hepatocellular carcinoma treatment: hurdles, advances, and prospects. Hepat Oncol. 2018;5(2):HEP08. https://doi.org/10.2217/hep-2018-0002.
Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. 2020;2(1):zcaa002. https://doi.org/10.1093/narcan/zcaa002.
Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3–19. https://doi.org/10.1158/1541-7786.
Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35(4): e00225. https://doi.org/10.1042/BSR20150089.
D’Amico L, Menzel U, Prummer M, Müller P, Buchi M, Kashyap A, et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumour immunity and potentiates PD-1 blockade in breast cancer. J Immunother Cancer. 2019;7(1):1–5. https://doi.org/10.1186/s40425-018-0464-1.
Gerber HP, Koehn FE, Abraham RT. The antibody-drug conjugate: an enabling modality for natural product-based cancer therapeutics. Nat Prod Rep. 2013;30(5):625–39. https://doi.org/10.1039/c3np20113a.
Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl. 2014;53(15):3796–827. https://doi.org/10.1002/anie.201307628.
Lambert JM, Morris CQ. Antibody-drug conjugates (ADCs) for personalized treatment of solid tumours: a review. Adv Ther. 2017;34(5):1015–35. https://doi.org/10.1007/s12325-017-0519-6.
Siracusano G, Tagliamonte M, Buonaguro L, Lopalco L. Cell Surface proteins in hepatocellular carcinoma: from bench to bedside. Vaccines. 2020;8(1):41. https://doi.org/10.3390/vaccines8010041.
Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett. 2017;3:1041–7. https://doi.org/10.3892/ol.2017.5557.
Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. https://doi.org/10.1016/j.ejpb.2015.03.018.
Schrama D, Reisfeld R, Becker J. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–59. https://doi.org/10.1038/nrd1957.
Richardson DL, et al. Antibody drug conjugates in the treatment of epithelial ovarian cancer. Hematol Oncol Clin North Am. 2018;32(6):1057–71. https://doi.org/10.1016/j.hoc.2018.07.014.
Nejadmoghaddam M-R, et al. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol. 2019;201911(1):3–23 (PMID: 30800238).
Nasiri H, et al. Antibody-drug conjugates: promising and efficient tools for targeted cancer therapy. J Cell Physiol. 2018;233(9):6441–57. https://doi.org/10.1002/jcp.26435.
Schroeder B, McNiven MA. Importance of endocytic pathways in liver function and disease. Compr Physiol. 2014;4(4):1403–17. https://doi.org/10.1002/cphy.c140001.
Tang H, Liu Y, Yu Z, Sun M, Lin L, Liu W, et al. The analysis of key factors related to adcs structural design. Front Pharmacol. 2019;24(10):373. https://doi.org/10.3389/fphar.2019.00373.
Kim EG, Kim KM. Strategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeutic. Biomol Ther. 2015;23(6):493–509. https://doi.org/10.4062/biomolther.2015.116.
Chen X, Ding G, Gao Q, Sun J, Zhang Q, Du L, et al. A human anti-c-Met Fab fragment conjugated with doxorubicin as targeted chemotherapy for hepatocellularcarcinoma. PLoS One. 2013;8(5):e63093. https://doi.org/10.1371/journal.pone.0063093.
Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br JCancer. 2008;99(1):100–9. https://doi.org/10.1038/sj.bjc.6604437.
Sun F, Wang Y, Luo X, Ma Z, Xu Y, Zhang X, et al. Anti-CD24 antibody–nitric oxide conjugate selectively and potently suppresses hepatic carcinoma. Cancer Res. 2019;79(13):3395–405. https://doi.org/10.1158/0008-5472.
Ma Z, He H, Sun F, Xu Y, Huang X, Ma Y, et al. Selective targeted delivery of doxorubicin via conjugating to anti-CD24 antibody results in enhanced antitumor potency for hepatocellular carcinoma both in vitro and in vivo. J Cancer Res Clin Oncol. 2017;143(10):1929–40. https://doi.org/10.1007/s00432-017-2436-0.
Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J. 2008;14(3):154–69. https://doi.org/10.1097/PPO.0b013e318172d704.
Rüker F, Wozniak-Knopp G, editors. Introduction to Antibody Engineering. Springer Nature; 2021.
Ma Y, Zhang M, Wang J, Huang X, Kuai X, Zhu X, et al. High-affinity human anti-c-Met IgG conjugated to oxaliplatin as targeted chemotherapy for hepatocellular carcinoma. Front Oncol. 2019;2(9):717. https://doi.org/10.3389/fonc.2019.00717.
Ribas A. Clinical development of the anti–CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450–4. https://doi.org/10.1053/j.seminoncol.
Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, et al. Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci. 2018;109(3):531–41. https://doi.org/10.1111/cas.13485.
Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20(4):460–70. https://doi.org/10.1016/j.coi.2008.06.012.
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33. https://doi.org/10.1111/j.1476-5381.2009.00190.x.
Zhang P, Shi B, Gao H, Jiang H, Kong J, Yan J, et al. An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression. Cancer Immunol Immunother. 2014;63(2):121–32. https://doi.org/10.1007/s00262-013-1497-4.
Krishnamurthy A, Jimeno A. Bispecific antibodies for cancer therapy: a review. Pharmacol Ther. 2018;1(185):122–34. https://doi.org/10.1016/j.pharmthera.2017.12.002.
Lu J, Jiang F, Lu A, Zhang G. Linkers having a crucial role in antibody-drug conjugates. Int J Mol Sci. 2016;17(4):561. https://doi.org/10.3390/ijms17040561.
Trail PA, Dubowchik GM, Lowinger TB. Antibody-drug conjugates for the treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharmacol Ther. 2018;181:126–42. https://doi.org/10.1016/j.pharmthera.2017.07.013.
Beaumont M, Tomazela D, Hodges D, Ermakov G, Hsieh E, Figueroa I, et al. Antibody-drug conjugates: integrated bioanalytical and disposition assessments in lead optimization and selection. AAPS Open. 2018;4(1):1–7. https://doi.org/10.1186/s41120-018-0026-0.
Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111(6):538–49. https://doi.org/10.1093/jnci/djz035.
Gupta N, Kancharla J, Kaushik S, Ansari A, Hossain S, Goyal R, et al. Development of a facile antibody-drug conjugate platform for increased stability and homogeneity. Chem Sci. 2018;8(3):2387–95. https://doi.org/10.1039/c6sc05149a (Epub 2016 Dec 9).
Kern JC, Cancilla M, Dooney D, Kwasnjuk K, Zhang R, Beaumont M, et al. Discovery of pyrophosphate diesters as tunable, soluble, and bioorthogonal linkers for site-specific antibody-drug conjugates. J Am Chem Soc. 2016;138(4):1430–45. https://doi.org/10.1021/jacs.5b12547.
Rossi C, Chrétien ML, Casasnovas RO. Antibody-drug conjugates for the treatment of hematological malignancies: a comprehensive review. Target Oncol. 2018;13(3):287–308. https://doi.org/10.1007/s11523-018-0558-1.
Staudacher AH, Brown MP. Antibody-drug conjugates and bystander killing: is antigen-dependent internalization required? Br J Cancer. 2017;117(12):1736–42. https://doi.org/10.1038/bjc.2017.367.
Walles M, Connor A, Hainzl D. ADME and safety aspects of non-cleavable linkers in drug discovery and development. Curr Top Med Chem. 2017;17(32):3463–75. https://doi.org/10.2174/1568026618666180118153502.
Pankowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody-drug conjugates for cancer therapy. MAbs. 2014;6(1):34–45. https://doi.org/10.4161/mabs.27022.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–37. https://doi.org/10.1038/nrd.2016.268.
Dan N, Setua S, Kashyap VK, Khan S, Jaggi M, Yallapu MM, et al. Antibody-drug conjugates for cancer therapy: chemistry to clinical implications. Pharmaceuticals. 2018;11(2):32. https://doi.org/10.3390/ph11020032.
Verma VA, Pillow TH, DePalatis L, Li G, Phillips GL, Polson AG, et al. The cryptophycins as potent payloads for antibody-drug conjugates. Bioorg Med Chem Lett. 2015;25(4):864–8. https://doi.org/10.1016/j.bmcl.2014.12.070.
Yaghoubi S, Karimi MH, Lotfinia M, Gharibi T, Mahi-Birjand M, Kavi E, et al. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol. 2020;235(1):31–64. https://doi.org/10.1002/jcp.28967.
Calo CA, O’Malley DM. Antibody-drug conjugates for the treatment of ovarian cancer. Expert Opin Biol Ther. 2020;7:1–3. https://doi.org/10.1080/14712598.2020.1776253.
Masters JC, Nickens DJ, Xuan D, Shazer RL, Amantea M. Clinical toxicity of antibody-drug conjugates: a meta-analysis of payloads. Invest New Drugs. 2018;36(1):121–35. https://doi.org/10.1007/s10637-017-0520-6.
Bhaduri S, Ranjan N, Arya DP. An overview of recent advances in duplex DNA recognition by small molecules. Beilstein J Org Chem. 2018;14(1):1051–86. https://doi.org/10.3762/bjoc.14.93.
Toader D. Antibody-Drug Conjugates. In: Waring MJ, editor. Cancer II. Topics in medicinal chemistry. Cham: Springer; 2017. p. 289. https://doi.org/10.1007/7355_2017_29.
Donaghy H. Effects of antibody, drug, and linker on the preclinical and clinical toxicities of antibody-drugconjugates. MAbs. 2016;8(4):659–71. https://doi.org/10.1080/19420862.2016.1156829.
Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13(11):2618–29. https://doi.org/10.1158/1535-7163.MCT-14-0040-T.
Govindan SV, Sharkey RM, Goldenberg DM. Prospects and progress of antibody-drug conjugates in solid tumor. Expert Opin Biol Ther. 2016;16(7):883–93. https://doi.org/10.1517/14712598.2016.1173203.
Fu Y, Urban DJ, Nani RR, Zhang YF, Li N, Fu H, et al. Glypican-3-specific antibody drug conjugates targeting hepatocellular carcinoma. Hepatology. 2019;70(2):563–76. https://doi.org/10.1002/hep.30326 (Epub 2019 Feb 19).
Rios-Doria J, Harper J, Rothstein R, Wetzel L, Chesebrough J, Marrero A, et al. Antibody-drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 2017;77(10):2686–98. https://doi.org/10.1158/0008-5472.CAN-16-2854.
Pearce MC, Gamble JT, Kopparapu PR, O’Donnell EF, Mueller MJ, Jang HS, et al. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget. 2018;9(40):26072. https://doi.org/10.18632/oncotarget.25437.
Wang J, Wu Z, Pan G, Ni J, Xie F, Jiang B, et al. Enhanced doxorubicin delivery to hepatocellular carcinoma cells via CD147 antibody-conjugated immunoliposomes. Nanomedicine. 2018;14(6):1949–61. https://doi.org/10.1016/j.nano.2017.09.012.
Dosio F, Brusa P, Cattel L. Immunotoxins and anticancer drug conjugate assemblies: the role of the linkage between components. Toxins. 2011;3(7):848–83. https://doi.org/10.3390/toxins3070848.
Cvitkovic E, Bekradda M. Oxaliplatin: a new therapeutic option in colorectal cancer. Semin Oncol. 1999;26(6):647–62 (PMID: 10606258).
Jiang T, Zhang H, Liu X, Song H, Yao R, Li J, et al. Effect of oxaliplatin combined with polyenephosphatidylcholine on the proliferation of human gastric cancer SGC-7901 cells. Oncol Lett. 2016;12(6):4538–46. https://doi.org/10.3892/ol.2016.5293.
Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semin Oncol. 2002;29(5):11–20. https://doi.org/10.1053/sonc.2002.35524.
Hickok JR, Thomas DD. Nitric oxide and cancer therapy: the emperor has NO clothes. Curr Pharm Des. 2010;16(4):381–91. https://doi.org/10.2174/138161210790232149.
Yasuda H, Yanagihara K, Nakayama K, Mio T, Sasaki T, Asada M et al. Therapeutic applications of nitric oxide for malignant tumor in animal models and human studies. InNitric Oxide (NO) and Cancer. 2010
Chen H, Lin Z, Arnst KE, Miller DD, Li W. Tubulin inhibitor-based antibody-drug conjugates for cancer therapy. Molecules. 2017;22(8):1281. https://doi.org/10.3390/molecules22081281.
Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394(10200):793–804. https://doi.org/10.1016/S0140-6736(19)31774-X.
Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17(6):e254–62. https://doi.org/10.1016/S1470-2045(16)30030-4.
Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R, et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther. 2010;9(10):2689–99. https://doi.org/10.1158/1535-7163.MCT-10-0644.
Baron JM, Boster BL, Barnett CM. Ado-trastuzumabemtansine (T-DM1): a novel antibody-drug conjugate for the treatment of HER2-positive metastatic breast cancer. J Oncol Pharm Pract. 2015;21(2):132–42. https://doi.org/10.1177/1078155214527144.
Trnĕný M, Verhoef G, Dyer MJ, Yehuda DB, Patti C, Canales M, et al. A phase II multicenter study of the anti-CD19 antibody-drug conjugate cetuximabravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica. 2018;103(8):1351. https://doi.org/10.3324/haematol.2017.168401.
Buecheler JW, Winzer M, Tonillo J, Weber C, Gieseler H. Impact of payload hydrophobicity on the stability of antibody-drug conjugates. Mol Pharm. 2018;15(7):2656–64. https://doi.org/10.1021/acs.molpharmaceut.8b00177.
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925. https://doi.org/10.1038/nbt.1480.
Chudasama V, Maruani A, Caddick S. Recent advances in the construction of antibody-drug conjugates. Nat Chem. 2016;8(2):114. https://doi.org/10.1038/nchem.2415.
Akkapeddi P, Azizi SA, Freedy AM, Cal PM, Gois PM, Bernardes GJ. Construction of homogeneous antibody-drug conjugates using site-selective protein chemistry. Chem Sci. 2016;7(5):2954–63. https://doi.org/10.1039/c6sc00170j.
Pysz I, Jackson PJM, Thurston DE. CHAPTER 1: introduction to antibody-drug conjugates (ADCs). In: Cytotoxic Payloads for antibody-drug conjugates. Cambridge: Royal Society of chemistry; 2019. p. 1–30. https://doi.org/10.1039/9781788012898-00001.
Cao M, De Mel N, Jiao Y, Howard J, Parthemore C, Korman S, et al. Site-specific antibody-drug conjugate heterogeneity characterization and heterogeneity root cause analysis. MAbs. 2019;11(6):1064–76. https://doi.org/10.1080/19420862.2019.1624127.
Coumans RG, Ariaans GJ, Spijker HJ, RenartVerkerk P, Beusker PH, Kokke BP, et al. A Platform for the generation of site-specific antibody-drug conjugates that allows for selective reduction of engineered cysteines. Bioconjugate Chem. 2020;31(9):2136–46. https://doi.org/10.1021/acs.bioconjchem.0c00337.
Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9(1):33–46. https://doi.org/10.1007/s13238-016-0323-0.
Walsh SJ, Bargh JD, Dannheim FM, Hanby AR, Seki H, Counsell AJ, et al. Site-selective modification strategies in antibody-drug conjugates. Chem Soc Rev. 2021;50:1305–53. https://doi.org/10.1039/D0CS00310G.
García-Vilas JA, Medina MÁ. Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J Gastroenterol. 2018;24(33):3695. https://doi.org/10.3748/wjg.v24.i33.3695.
Wang H, Rao B, Lou J, Li J, Liu Z, Li A, et al. The function of the HGF/c-Met axis in hepatocellular carcinoma. Front Cell Dev Biol. 2020;7(8):55. https://doi.org/10.3389/fcell.2020.00055.
Petrini I. Biology of MET: a double life between normal tissue repair and tumor progression. Ann Transl Med. 2015;3(6):82. https://doi.org/10.3978/j.issn.2305-5839.2015.03.58.
Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21(41):11673. https://doi.org/10.3748/wjg.v21.i41.11673.
Oku Y, Shimoji T, Takifuji K, Hotta T, Yokoyama S, Matsuda K, et al. Identification of the molecular mechanisms for dedifferentiation at the invasion front of colorectal cancer by a gene expression analysis. Clin Cancer Res. 2008;14(22):7215–22. https://doi.org/10.1158/1078-0432.CCR-08-0370.
Venepalli NK, Goff L. Targeting the HGF-cMET axis in hepatocellular carcinoma. Int J Hepatol. 2013;2013:1–11. https://doi.org/10.1155/2013/341636.
Papaccio F, Della Corte CM, Viscardi G, Di Liello R, Esposito G, Sparano F, et al. HGF/MET, and the immune system: relevance for cancer immunotherapy. Int J Mol Sci. 2018;19(11):3595. https://doi.org/10.3390/ijms19113595.
Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30(1):3–11. https://doi.org/10.1055/s-0033-1333648.
Fiume L, Bolondi L, Busi C, Chieco P, Kratz F, Lanza M, et al. Doxorubicin coupled to lactosaminated albumin inhibits the growth of hepatocellular carcinomas induced in rats by diethylnitrosamine. J Hepatol. 2005;43(4):645–52. https://doi.org/10.1016/j.jhep.2005.02.045.
Yoo HS, Lee EA, Park TG. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J Control Release. 2002;82(1):17–27.
Johnson-Arbor K, Dubey R. Doxorubicin, In: StatPearls, Treasure Island: StatPearls Publishing; 2021. PMID: 29083582
Cox J, Weinman S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat Oncol. 2016;3(1):57–9. https://doi.org/10.1016/s0168-3659(02)00088-3.
Kalim M, Chen J, Wang S, Lin C, Ullah S, Liang K, et al. Intracellular trafficking of new anticancer therapeutics: antibody-drug conjugates. Drug Des Devel Ther. 2017;11:2265. https://doi.org/10.2147/DDDT.S135571.
Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1–30. https://doi.org/10.1186/s12929-019-0592-z.
Fu XT, Song K, Zhou J, Shi YH, Liu WR, Shi GM, et al. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019;19(1):1–1. https://doi.org/10.1186/s12935-019-0771-8.
Salomon PL, Reid EE, Archer KE, Harris L, Maloney EK, Wilhelm AJ, et al. Optimizing lysosomal activation of antibody-drug conjugates (ADCs) by incorporation of novel cleavable dipeptide linkers. Mol Pharm. 2019;16(12):4817–25. https://doi.org/10.1021/acs.molpharmaceut.9b00696.
Qin S, Wu Q. Systemic chemotherapy with oxaliplatin is a good option for advanced hepatocellular carcinoma. Hepat Oncol. 2015;2(3):203–7. https://doi.org/10.2217/hep.15.14.
Li L, Chen J, Ge C, Zhao F, Chen T, Tian H, et al. CD24 isoform promotes cell proliferation, migration, and invasion and is downregulated by EGR1 in hepatocellular carcinoma. Onco Targets Ther. 2019;12:1705. https://doi.org/10.2147/OTT.S196506.
Chen Z, Wang T, Tu X, Xie W, He H, Wang M, et al. Antibody-based targeting of CD24 enhances the antitumor effect of cetuximab via attenuating phosphorylation of Src/STAT3. Biomed Pharmacother. 2017;1(90):427–36. https://doi.org/10.1016/j.biopha.2017.03.094.
Ryu J, Kang M, Lee MS, Kim HJ, Nam SH, Song HE, et al. Cross talk between the TM4SF5/focal adhesion kinase and the interleukin-6/STAT3 pathways promotes immune escape of human liver cancer cells. Mol Cell Biol. 2014;34(16):2946–60. https://doi.org/10.1128/MCB.00660-14.
Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24+ liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9(1):50–63. https://doi.org/10.1016/j.stem.2011.06.005.
Yin SS, Gao FH. Molecular mechanism of tumor cell immune escape mediated by CD24/siglec-10. Front Immunol. 2020;17(11):1324. https://doi.org/10.3389/fimmu.2020.01324.
Chinje EC, Stratford IJ. Role of nitric oxide in the growth of solid tumours: a balancing act. Essays Biochem. 1997;1(32):61–72 (PMID: 9493011).
Yongsanguanchai N, Pongrakhananon V, Mutirangura A, Rojanasakul Y, Chanvorachote P. Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am J Physiol Cell Physiol. 2015;308(2):C89-100. https://doi.org/10.1152/ajpcell.00187.2014.
Zhang X, Jin L, Tian Z, Wang J, Yang Y, Liu J, et al. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6398894/ Cancer Sci. 2019;110(3):1054–63. https://doi.org/10.1111/cas.13945.
Weiming XU, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12(5):311–20. https://doi.org/10.1038/sj.cr.7290133.
Salnikov AV, Bretz NP, Perne C, Hazin J, Keller S, Fogel M, et al. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br J Cancer. 2013;108(7):1449–59. https://doi.org/10.1038/bjc.2013.102.
Chakravarthi S, Jessop CE, Bulleid NJ. The role of glutathione in disulfide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006;7(3):271–5. https://doi.org/10.1038/sj.embor.7400645.
Ma Z, He H, Sun F, Xu, , et al. Selective targeted delivery of doxorubicin via conjugating to anti-CD24 antibody results in enhanced antitumor potency for hepatocellular carcinoma both in vitro and in vivo. J Cancer Res Clin Oncol. 2017;143(10):1929–40. https://doi.org/10.1007/s00432-017-2436-0.
Sun F, Wang T, Jiang J, Wang Y, Ma Z, Li Z, et al. Engineering a high-affinity humanized anti-CD24 antibody to target hepatocellular carcinoma by a novel CDR grafting design. Oncotarget. 2017;8(31):51238. https://doi.org/10.18632/oncotarget.17228.
Meng J, Sun B, Zhao X, Zhang D, Zhao X, Gu Q, et al. Doxycycline as an inhibitor of the epithelial-to-mesenchymal transition and vasculogenic mimicry in hepatocellular carcinoma. Mol Cancer Ther. 2014;13(12):3107–22. https://doi.org/10.1158/1535-7163.MCT-13-1060.
Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: a new target for diagnosis and treatment of hepatocellular carcinoma. J Cancer. 2020;11(8):2008. https://doi.org/10.7150/jca.39972.
Chouhan S, Singh S, Athavale D, Ramteke P, Pandey V, Joseph J, et al. Glucose induced activation of canonical Wnt signaling pathway in hepatocellular carcinoma is regulated by DKK4. Sci Rep. 2016;6(1):1–5. https://doi.org/10.1038/srep27558.
Chouhan S, Singh S, Athavale D, Ramteke P, Vanuopadath M, Nair BG, et al. Sensitization of hepatocellular carcinoma cells towards doxorubicin and sorafenib is facilitated by glucose-dependent alterations in reactive oxygen species, P-glycoprotein and DKK4. J Biosci. 2020;45(1):1–23 (PMID: 32713860).
Nishida T, Kataoka H. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers. 2019;11(9):1339. https://doi.org/10.3390/cancers11091339.
Wang S, Chen N, Chen Y, Sun L, Li L, et al. Elevated GPC3 level promotes cell proliferation in liver cancer. Oncol Lett. 2018;16(1):970–6. https://doi.org/10.3892/ol.2018.8754.
Lai JP, Sandhu DS, Yu C, Han T, Moser CD, Jackson KK, et al. Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 2008;47(4):1211–22. https://doi.org/10.1002/hep.22202.
Yu M, Luo H, Fan M, Wu X, Shi B, Di S, et al. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther. 2018;26(2):366–78. https://doi.org/10.1016/j.ymthe.2017.12.012.
Park JO, Stephen Z, Sun C, Veiseh O, Kievit FM, Fang C, et al. Glypican-3 targeting of liver cancer cells using multifunctional nanoparticles. Mol imaging. 2011;10(1):69–77 (PMID: 21303616).
Ortiz MV, Roberts SS, Glade Bender J, Shukla N, Wexler LH. Immunotherapeutic targeting of GPC3 in pediatric solid embryonal tumors. Front Oncol. 2019;26(9):108. https://doi.org/10.3389/fonc.2019.00108.
Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47(3):333–8. https://doi.org/10.1016/j.ejca.2010.10.024.
Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, et al. Antibody-drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res. 2019;25(18):5441–8. https://doi.org/10.1158/1078-0432.CCR-19-0272.
Li N, Gao W, Zhang YF, Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018;4(11):741–54. https://doi.org/10.1016/j.trecan.2018.09.004.
Zhang YF, Ho M. Humanization of high-affinity antibodies targeting glypican-3 in hepatocellular carcinoma. Sci Rep. 2016;6(1):1–1. https://doi.org/10.1038/srep33878.
Lohitesh K, Chowdhury R, Mukherjee S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight. Cancer Cell Int. 2018;18(1):1–5. https://doi.org/10.1186/s12935-018-0538-7.
Patil CP, Satam Lee VM. A short review on the synthetic strategies of duocarmycin analogs that are powerful DNA alkylating agents. Anticancer Agents Med Chem. 2015;15(5):616–30. https://doi.org/10.2174/1871520615666141216144116.
Diamantis N, Banerji U. Antibody-drug conjugates—an emerging class of cancer treatment. Br J Cancer. 2016;114(4):362–7. https://doi.org/10.1038/bjc.2015.435.
Pillow TH, Tercel M. Duocarmycin–PBD Dimers as Antibody–Drug Conjugate (ADC) Payloads. In: Thurston DE, Jackson PJM, editors. Cytotoxic Payloads for Antibody–Drug Conjugates. Royal Society of Chemistry; 2019. pp. 241–258.
Anami Y, Yamazaki CM, Xiong W, Gui X, Zhang N, An Z, et al. Glutamic acid–valine–citrulline linkers ensure stability and efficacy of antibody-drug conjugates in mice. Nat Commun. 2018;9(1):1–9. https://doi.org/10.1038/s41467-018-04982-3.
Hartley JA. The development of pyrrolobenzodiazepines as antitumour agents. Expert Opin Investig Drugs. 2011;20(6):733–44. https://doi.org/10.1517/13543784.2011.573477 (Epub 2011 Apr 4).
Marcucci F, Caserta CA, Romeo E, Rumio C. Antibody-drug conjugates (ADC) against cancer stem-like cells (CSC)—is there still room for optimism? Front Oncol. 2019;9:167. https://doi.org/10.3389/fonc.2019.00167.
Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016;159(5):481–90. https://doi.org/10.1093/jb/mvv127.
Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95(10):1371–8. https://doi.org/10.1038/sj.bjc.6603425.
Heinzmann D, Noethel M, Ungern-Sternberg SV, Mitroulis I, Gawaz M, Chavakis T, et al. CD147 is a novel interaction partner of integrin αMβ2 mediating leukocyte and platelet adhesion. Biomolecules. 2020;10(4):541. https://doi.org/10.3390/biom10040541.
Shang YK, Li C, Liu ZK, Kong LM, Wei D, Xu J, et al. System analysis of the regulation of the immune response by CD147 and FOXC1 in cancer cell lines. Oncotarget. 2018;9(16):12918. https://doi.org/10.18632/oncotarget.24161.
Kendrick AA, Schafer J, Dzieciatkowska M, Nemkov T, D’Alessandro A, Neelakantan D, et al. CD147: a small molecule transporter ancillary protein at the crossroad of multiple hallmarks of cancer and metabolic reprogramming. Oncotarget. 2017;8(4):6742. https://doi.org/10.18632/oncotarget.14272.
Li X, Zhang Y, Ma W, Fu Q, Liu J, Yin G, et al. Enhanced glucose metabolism mediated by CD147 contributes to immunosuppression in hepatocellular carcinoma. Cancer Immunol Immunother. 2020;21:1–4. https://doi.org/10.1007/s00262-019-02457-y.
Vanherwegen AS, Gysemans C, Overbergh L. Dendritic cell metabolism: immunity and tolerance. Oncotarget. 2015;6(33):34039. https://doi.org/10.18632/oncotarget.5865.
Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol. 2019;24(10):2278. https://doi.org/10.3389/fimmu.2019.02278.
Li J, Huang Q, Long X, Zhang J, Huang X, Aa J, et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol. 2015;63(6):1378–89. https://doi.org/10.1016/j.jhep.2015.07.039.
Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Method Enzymol. 2005;1(391):71–97. https://doi.org/10.1016/s0076-6879(05)91004-5.
Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42(12):742 (PMID: 29234213).
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;1(6):286. https://doi.org/10.3389/fphar.2015.00286.
Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):1–4. https://doi.org/10.1186/s40169-018-0198-1.
Maeda K, Ding Q, Yoshimitsu M, Kuwahata T, Miyazaki Y, Tsukasa K, et al. CD133 modulate HIF-1α expression under hypoxia in EMT phenotype pancreatic cancer stem-like cells. Int J Mol Sci. 2016;17(7):1025. https://doi.org/10.1186/s40169-018-0198-1.
Bauer N, Fonseca AV, Florek M, Freund D, Jászai J, Bornhäuser M, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs. 2008;188(1–2):127–38. https://doi.org/10.1159/000112847.
Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351(4):820–4. https://doi.org/10.1016/j.bbrc.2006.10.128.
Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–58. https://doi.org/10.1038/sj.onc.1210811.
Wang N, Wang S, Li MY, Hu BG, Liu LP, Yang SL, et al. Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies. Ther Adv Med Oncol. 2018;10:1758835918816287. https://doi.org/10.1177/1758835918816287.
Dai X, Guo Y, Hu Y, Bao X, Zhu X, Fu Q, et al. Immunotherapy for targeting cancer stem cells in hepatocellular carcinoma. Theranostics. 2021;11(7):3489. https://doi.org/10.7150/thno.54648.
Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M, Gentile R, et al. Antibody-drug conjugates: the new frontier of chemotherapy. Int J Mol Sci. 2020;21(15):5510. https://doi.org/10.3390/ijms21155510.
Yaghoubi S, Karimi MH, Lotfinia M, Gharibi T, Mahi-Birjand M, Kavi E, Hosseini F, Sineh Sepehr K, Khatami M, Bagheri N, Abdollahpour-Alitappeh M. Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol. 2020;235(1):31–64. https://doi.org/10.1002/jcp.28967.
Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani AH. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol. 2019;11(1):3. https://doi.org/10.1080/19420862.2021.
Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody–drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3–19. https://doi.org/10.1158/1541-7786.
Lin JH, Guo Y, Wang W. Challenges of antibody drug conjugates in cancer therapy: current understanding of mechanisms and future strategies. Curr Pharmacol Rep. 2018;4(1):10–26. https://doi.org/10.1007/s40495-018-0122-9.
Leopold LH, Berger MS, Feingoldr J. Acute and long-term toxicities associated with gemtuzumab ozogamicin (mylotarg®) therapy of acute myeloid leukemia. Clin Lymphoma. 2002;1(2):S29-34. https://doi.org/10.3816/CLM.2002.s.006.
Rajvanshi P, Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood J Am Soc Hematol. 2002;99(7):2310–4. https://doi.org/10.1182/blood.V99.7.2310.
Gorovits B, Krinos-Fiorotti C. Proposed mechanism of off-target toxicity for antibody–drug conjugates driven by mannose receptor uptake. Cancer Immunol Immunother. 2013;62(2):217–23. https://doi.org/10.1007/s00262-012-1369-3.
LoRusso PM, Lomen PL, Redman BG, Poplin E, Bander JJ, Valdivieso M. Phase I study of monoclonal antibody-ricin A chain immunoconjugate Xomazyme-791 in patients with metastatic colon cancer. Am J Clin Oncol. 1995;18(4):307–12. https://doi.org/10.1097/00000421-199508000-00008.
Winer LM, O’Dwyer J, Kitson J, Comis RL, Frankel AE, Bauer RJ, Konrad MS, Groves ES. Phase I evaluation of an anti-breast carcinoma monoclonal antibody 260F9-recombinant ricin A chain immunoconjugate. Can Res. 1989;49(14):4062–7.
Kim YW, Chang TW. Potential use of immunoconjugates for AIDS therapy. AIDS Res Hum Retroviruses. 1992;8(6):1033–8.
Skilleter DN, Price RJ, Parnell GD, Cumber AJ. The low uptake of an abrin A-chain immunotoxin by rat hepatic cells in vivo and in vitro. Cancer Lett. 1989;46(3):161–6.
Mosure KW, Henderson AJ, Klunk LJ, Knipe JO. Disposition of conjugate-bound and free doxorubicin in tumor-bearing mice following administration of a BR96-doxorubicin immunoconjugate (BMS 182248). Cancer Chemother Pharmacol. 1997;40(3):251–8.
Herbertson RA, Tebbutt NC, Lee FT, MacFarlane DJ, Chappell B, Micallef N, Lee ST, Saunder T, Hopkins W, Smyth FE, Wyld DK. Phase I biodistribution and pharmacokinetic study of Lewis Y–targeting immunoconjugate CMD-193 in patients with advanced epithelial cancers. Clin Cancer Res. 2009;15(21):6709–15.
Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T, Jonak ZL. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol. 2003;21(2):211–22.
Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blättler WA, Goldmacher VS. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Can Res. 2006;66(6):3214–21.
Collins DM, Bossenmaier B, Kollmorgen G, Niederfellner G. Acquired resistance to antibody-drug conjugates. Cancers. 2019;11(3):394.
García-Alonso S, Ocaña A, Pandiella A. Resistance to antibody–drug conjugates. Can Res. 2018;78(9):2159–65.
Walter RB, Gooley TA, Van Der Velden VH, Loken MR, Van Dongen JJ, Flowers DA, Bernstein ID, Appelbaum FR. CD33 expression and P-glycoprotein–mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109(10):4168–70.
Matsui H, Takeshita A, Naito K, Shinjo K, Shigeno K, Maekawa M, Yamakawa Y, Tanimoto M, Kobayashi M, Ohnishi K, Ohno R. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia. 2002;16(5):813–9.
Rosen DB, Harrington KH, Cordeiro JA, Leung LY, Putta S, Lacayo N, Laszlo GS, Gudgeon CJ, Hogge DE, Hawtin RE, Cesano A. AKT signaling as a novel factor associated with in vitro resistance of human AML to gemtuzumab ozogamicin. PLoS One. 2013;8(1):e53518.
Donato EM, Fernández-Zarzoso M, Hueso JA, de la Rubia J. Brentuximab vedotin in Hodgkin lymphoma and anaplastic large-cell lymphoma: an evidence-based review. Onco Targets Ther. 2018;11:4583.
Acknowledgements
We acknowledge the support from Amrita VishwaVidyapeetham. We express our sincere gratitude to Dr Shanthikumar. V. Nair, Dean of Research, Amrita Vishwa Vidyapeetham and Dr Sabitha M, Principal, Amrita School of Pharmacy for the encouragement, support and all the facilities provided.
Funding
We acknowledge the support of the Amrita Vishwa Vidyapeetham PG student fellowship to MM. The work is partially supported by Amrita Vishwa Vidyapeetham faculty SEED grant to LRN (K-PHAR-20-627).
Author information
Authors and Affiliations
Contributions
LRN designed, conceptualized and writing the review. PK revised and proofread the manuscript. MM, ARK and ARD carried out a literature survey and writing. BLN and GP contributed to the artwork.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Research involving human participants and/or animals
ot applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have given their consent for the publishing of this manuscript.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murali, M., Kumar, A.R., Nair, B. et al. Antibody–drug conjugate as targeted therapeutics against hepatocellular carcinoma: preclinical studies and clinical relevance. Clin Transl Oncol 24, 407–431 (2022). https://doi.org/10.1007/s12094-021-02707-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02707-5