Abstract
Objectives
High-risk human papillomavirus (HR-HPV) is an important risk factor for esophageal cancer. Macrophages constitute a crucial immune medium for regulating HPV-related tumors; however, the specific regulatory mechanisms remain unknown. Therefore, the purpose of our current study was to investigate the mechanism by which HPV16E6 regulates macrophages to promote the invasion and metastasis of esophageal cancer.
Methods
HPV16E6 infection was detected by polymerase chain reaction. Immunohistochemistry was used to verify the distribution of tumor-associated macrophages (TAMs) and MMP-9 expression in esophageal squamous cell carcinoma tissues (ESCCs), and cancer adjacent normal tissues (CANs) from Kazakh patients. ESCC cells were transfected with a plasmid over-expressing HPV16E6 and non-contact cocultured with macrophages.
Results
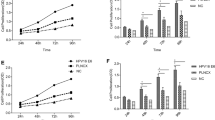
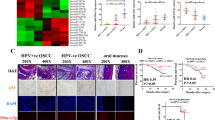
The infection rate of HPV16E6 in Kazakh ESCCs was clearly higher than that in CANs (P < 0.05). The density of CD163-positive TAMs was significantly positively correlated with HPV16E6 infection in ESCCs (P < 0.05). After coculturing macrophages and EC9706 cells transfected with the HPV16E6 plasmid, the phenotype of macrophages transformed into M2 macrophages. The migration and invasion ability of ESCC cells were higher in the HPV16E6-transfected and coculture group than in the HPV16E6 empty vector-transfected and non-cocultured HPV16E6-transfected groups (all P < 0.05). The density of M2-like TAMs in ESCCs was positively correlated with the level of MMP-9 expression. MMP-9 expression in the HPV16E6-ESCC coculture macrophages group was substantially higher than that in controls (all P < 0.05).
Conclusions
HPV16 infection mediates tumor-associated macrophages to promote ESCC invasion and migration.







Similar content being viewed by others
Availability of data and materials
The authors declare that all original data are available for inspection and evaluation.
References
Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Ekman S, Dreilich M, Lennartsson J, et al. Esophageal cancer: current and emerging therapy modalities. Expert Rev Anticancer Ther. 2008;8:1433–48.
Hu J, Li L, Pang L, et al. HLA-DRB1*1501 and HLA-DQB1*0301 alleles are positively associated with HPV16 infection–related Kazakh esophageal squamous cell carcinoma in Xinjiang China. Cancer Immunol Immunother. 2012;61:2135–41.
Zhou Y, Pan Y, Zhang S, et al. Increased phosphorylation of p70 S6 kinase is associated with HPV16 infection in cervical cancer and esophageal cancer. Br J Cancer. 2007;97:218–22.
Guang-Cheng D, Li-Dong W, Shao-Hua LI, et al. Correlation between human papillomavirus infection and esophageal squamous cell carcinoma and gastric cardia adenocarcinoma in high-incidence area of Henan province. Chinese J Cancer Prev Treat. 2009;16:252–5.
Kang XD, Zheng Y, Chen WG, et al. Effects of HPV16E6 transfection on the biological behavior of Eca109 and Eca9706 cells. Oncol Lett. 2018;15:1646–54.
Barros MR, de Melo CML, Rego MLCMG, et al. Activities of stromal and immune cells in HPV-related cancers. J Exp Clin Cancer Res. 2018;37(1):137.
Ilir A , Chen Z, Wang T, et al. Oral alpha, beta, and gamma HPV types and risk of incident esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(10):1168–75.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2008;9:239–52.
Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117:1583–91.
El-Arabey AA, Abdalla M, Abd-Allah AR. SnapShot: TP53 status and macrophages infiltration in TCGA-analyzed tumors. Int Immunopharmacol. 2020;86:106758.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64.
Geissmann F, Gordon S, Hume DA, et al. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–60.
Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–61.
Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337–49.
Hu JM, Liu K, Liu JH, et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squmous cell carcinoma. Oncotarget. 2017;8:21526–38.
Guo SJ, Lin DM, Li J, et al. Tumor-associated macrophages and CD3-zeta expression of tumor-infiltrating lymphocytes in human esophageal squamous-cell carcinoma. Dis Esophagus. 2007;20(2):107–16.
Verollet C, Charriere GM, Labrousse A, et al. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur J Immunol. 2011;41:2805–13.
Spector TD, et al. Risk factors for osteoarthritis: genetics. Osteoarthr Cartil. 2004;12:S39–44.
Wang K, Zhou Y, Li G, et al. MMP8 and MMP-9 gene polymorphisms were associated with breast cancer risk in a Chinese Han population. Sci Rep. 2018;8:13422.
Chu D, Zhao Z, Yi Z, et al. Matrix metalloproteinase-9 is associated with relapse and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2012;19:318–25.
Hwang TL, Lee LY, Wang CC, et al. Claudin-4 expression is associated with tumor invasion, MMP-2 and MMP-9 expression in gastric cancer. Exp Ther Med. 2010;1:789–97.
El-Arabey AA, Denizli M, Kanlikilicer P, et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal. 2020;68:109539.
Xiao-Jing C, Ling-Fei H, Xiang-Guang W, et al. Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J Cancer. 2017;8:3868–75.
Hu JM, Liu K, Liu JH, et al. The increased number of tumor-associated macrophage is associated with overexpression of VEGF-C, plays an important role in Kazakh ESCC invasion and metastasis. Exp Mol Pathol. 2017;102:15–22.
Jihong L, Chunxiao Li, Liyan Z, et al. Association of tumour-associated macrophages with cancer cell EMT, invasion, and metastasis of Kazakh oesophageal squamous cell cancer. Diagn Pathol. 2019;14:55.
Singh C, Roy-Chowdhuri S. Quantitative real-time PCR: recent advances. Methods Mol Biol. 2016;1392:161–76.
Marshall J. Transwell invasion assays. Methods Mol Biol. 2011;769:97–110.
Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;38:400–12.
Wang LD, Zhou FY, Li XM, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42(9):759–63.
Wang XL, Liu K, Liu JH, et al. High infiltration of CD68-tumor associated macrophages, predict poor prognosis in Kazakh esophageal cancer patients. Int J Clin Exp Pathol. 2017;10:10282–92.
Hu JM, Li L, Chen YZ, et al. Human papillomavirus type 16 infection may be involved in esophageal squamous cell carcinoma carcinogenesis in Chinese Kazakh patients. Dis Esophagus. 2013;26:703–7.
Cheah P-L, Koh C-C, et al. Esophageal squamous cell carcinomas in a Malaysian cohort show a lack of association with human papillomavirus. J Dig Dis. 2018;19:272–8.
Hosnjak L, Poljak M. A systematic literature review of studies reporting human papillomavirus (HPV) prevalence in esophageal carcinoma over 36 years (1982–2017). Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:127–36.
Bognar L, Hegedus I, Bellyei S, et al. Prognostic role of HPV infection in esophageal squamous cell carcinoma. Infect Agents Cancer. 2018;13(1):38–46.
Jaehong K, Jong-Sup B. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:1–11.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7.
Sigrun S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9:254.
Medrek C, Ponten F, Jirstrom K, et al. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306.
Subbarayan RS, Arnold L, Gomez JP, et al. The role of the innate and adaptive immune response in HPV-associated oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2019;4:508–12.
Li J, Xie Y, Wang X, et al. Overexpression of VEGF-C and MMP-9 predicts poor prognosis in Kazakh patients with esophageal squamous cell carcinoma. Peer J. 2019;7:8182–96.
Pelekanou V, Villarroel-Espindola F, Schalper KA, et al. CD68, CD163, and matrix metalloproteinase 9 (MMP-9) co-localization in breast tumor microenvironment predicts survival differently in ER-positive and -negative cancers. Breast Cancer Res. 2018;20:154.
Legrand C, Gilles C, Zahm J-M, et al. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol. 1999;146(2):517–29.
El-Arabey AA, Abdalla M, Abd-Allah AR. GATA3 and stemness of high-grade serous ovarian carcinoma: novel hope for the deadliest type of ovarian cancer. Hum Cell. 2020;33(3):904–6.
Lurier EB, Dalton D, Dampier W, et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology. 2017;222:847–56.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NO.81760428, NO.81960435 and NO.81460363), Start-up Project of High-level Talents Scientific Research in Shihezi University (RCZK2018C19), Science and Technology Development Project of Xinjiang Production and Construction Corps (NO. 2018AB033), National Early Detection and Treatment Project for Upper Digestive Tract in Rural Area in China (NO.2018), and The Youth Science and Technology Innovation Leading Talents Project of Corps (NO.2017CB004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2021_2642_MOESM1_ESM.docx
Supplementary file1 Table S1. Analyses of the clinical pathological parameters in ESCC patients. Table S2. The PCR reaction system of β-globin. Table S3. The PCR reaction system of the HPV16E6 gene. Table S4. The reaction system of qRT-PCR. Table S5. The reaction procedure of qRT-PCR (DOCX 46 KB)
Rights and permissions
About this article
Cite this article
Yuan, X., Liu, K., Li, Y. et al. HPV16 infection promotes an M2 macrophage phenotype to promote the invasion and metastasis of esophageal squamous cell carcinoma. Clin Transl Oncol 23, 2382–2393 (2021). https://doi.org/10.1007/s12094-021-02642-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02642-5