Abstract
Purpose
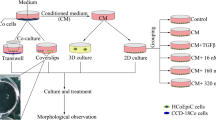
Although recent studies have suggested that neutral endopeptidase (NEP) is implicated in the regulation of colon cancer (CC) cell growth and metastasis, the influence of the tumor microenvironment on this role of NEP has not been investigated so far. Normal colon fibroblasts (NCFs) constitute a component of the stroma surrounding a tumor in an early stage of its development. NCFs can influence transformed cells via different paracrine factors, including TGF-β1. This in vitro study was undertaken to evaluate the role of NEP in CC promotion in conditions of indirect co-culture of CC cells (LS180 and SW620) with NCFs (CCD-18Co) or their conditioned medium (CM-18Co).
Methods
We examined cell proliferation (with the BrdU assay) and invasiveness (using BME-coated inserts, 8 µm) of NEP-expressing, NEP-silenced (siRNA), and NEP-inhibited (with thiorphan, i.e. a NEP specific inhibitor) CC cells cultured alone or co-cultured with CCD-18Co or with their conditioned medium. The Western blot and ELISA methods were used to assess the level of TGF-β1.
Results
The results showed that the co-culture of the NEP-depleted CC cells with NCFs or their conditioned medium resulted in a significant decrease in cell proliferation in comparison with the proliferative potential of NEP-silenced/inhibited CC cells cultivated alone. In contrast, the NEP depletion did not influence the invasiveness of CC cells in the co-cultures. The co-culture of CC cells with CCD-18Co or CM-C18Co resulted in increased synthesis of TGF-β1, while the NEP downregulation decreased the synthesis of TGF-β1 in CC cells and abolished the stimulatory effect of the co-cultures on TGF-β1 production.
Conclusions
The results suggest that the expression of NEP by colon cancer cells is essential for their proliferation and TGF-β1 synthesis during paracrine interactions with NCFs.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017 [cited 2019 Oct 9];66:683–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26818619
Belli C, Trapani D, Viale G, D’Amico P, Duso BA, Della Vigna P, et al. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018 [cited 2019 Oct 7];65:22–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0305737218300148
Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016 [cited 2019 Oct 8];99:186–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26278673
Alkasalias T, Moyano-Galceran L, Arsenian-Henriksson M, Lehti K. Fibroblasts in the Tumor Microenvironment: Shield or Spear? Int J Mol Sci. Multidisciplinary Digital Publishing Institute; 2018 [cited 2020 Apr 8];19:1532. Available from: http://www.mdpi.com/1422-0067/19/5/1532
Carl-McGrath S, Lendeckel U, Ebert M, Röcken C. Ectopeptidases in tumour biology: a review. Histol Histopathol. 2006 [cited 2019 Oct 9];21:1339–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16977585
Maguer-Satta V, Besançon R, Bachelard-Cascales E. Concise Review: Neutral Endopeptidase (CD10): A Multifaceted Environment Actor in Stem Cells, Physiological Mechanisms, and Cancer. Stem Cells. 2011 [cited 2019 Oct 9];29:389–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21425402
Sato Y, Itoh F, Hinoda Y, Ohe Y, Nakagawa N, Ueda R, et al. Expression of CD10/neutral endopeptidase in normal and malignant tissues of the human stomach and colon. J Gastroenterol. 1996 [cited 2019 Oct 10];31:12–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8808423
Ogawa H, Iwaya K, Izumi M, Kuroda M, Serizawa H, Koyanagi Y, et al. Expression of CD10 by stromal cells during colorectal tumor development. Hum Pathol. 2002 [cited 2019 Oct 10];33:806–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12203213
Oliveira LA de, Artigiani Neto R, Joppert Netto G, Sznirer M, Fernandes LC, Lima F de O, et al. Tissue expression of CD10 protein in colorectal carcinoma: correlation with the anatomopathological features of the tumor and with lymph node and liver metastases. J Coloproctology (Rio Janeiro). Sociedade Brasileira de Coloproctologia; 2012 [cited 2019 Oct 10];32:34–9. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S2237-93632012000100005&lng=en&nrm=iso&tlng=en
Fujimoto Y, Nakanishi Y, Sekine S, Yoshimura K, Akasu T, Moriya Y, et al. CD10 Expression in Colorectal Carcinoma Correlates With Liver Metastasis. Dis Colon Rectum. 2005 [cited 2019 Oct 10];48:1883–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16175325
Fujita S, Taniguchi H, Yao T, Shimoda T, Ueno H, Hirai T, et al. Multi-institutional study of risk factors of liver metastasis from colorectal cancer: correlation with CD10 expression. Int J Colorectal Dis. 2010 [cited 2019 Oct 11];25:681–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20204382
Kuniyasu H, Luo Y, Fujii K, Sasahira T, Moriwaka Y, Tatsumoto N, et al. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut. 2010 [cited 2019 Oct 9];59:348–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19828468
Raposo TP, Comes MS, Idowu A, Agit B, Hassall J, Fadhil W, et al. CD10 inhibits cell motility but expression is associated with advanced stage disease in colorectal cancer. Exp Mol Pathol. 2018 [cited 2019 Oct 9];104:190–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0014480017306500
Mizerska-Kowalska M, Bojarska-Junak A, Jakubowicz-Gil J, Kandefer-Szerszeń M. Neutral endopeptidase (NEP) is differentially involved in biological activities and cell signaling of colon cancer cell lines derived from various stages of tumor development. Tumor Biol. 2016 [cited 2019 Oct 9];37:13355–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27460083
Mizerska-Kowalska M, Kreczko-Kurzawa J, Zdzisińska B, Czerwonka A, Sławińska-Brych A, Maćkiewicz Z, et al. Neutral endopeptidase (NEP) inhibitors: thiorphan, sialorphin, and its derivatives exert anti-proliferative activity towards colorectal cancer cells in vitro. Chem Biol Interact. 2019 [cited 2019 Oct 9];307:105–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31054283
Liao Z, Tan ZW, Zhu P, Tan NS. Cancer-associated fibroblasts in tumor microenvironment: accomplices in tumor malignancy. Cell Immunol. 2019 [cited 2019 Oct 7];343:103729. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29397066
Meulmeester Erik ten DP. The dynamic roles of TGF-β in cancer. J Pathol. 2011 [cited 2019 Oct 7];223:2015–218. Available from: https://onlinelibrary.wiley.com/doi/pdf/https://doi.org/10.1002/path.2785
Costanza B, Umelo I, Bellier J, Castronovo V, Turtoi A. Stromal Modulators of TGF-β in Cancer. J Clin Med. 2017 [cited 2019 Oct 8];6:7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28067804
Xu Y, Pasche B. TGF-β signaling alterations and susceptibility to colorectal cancer. Hum Mol Genet. Oxford Academic; 2007 [cited 2020 Nov 16];16:R14–20. Available from: http://academic.oup.com/hmg/article/16/R1/R14/2355940/TGFβ-signaling-alterations-and-susceptibility-to
Gonzalez-Zubeldia I, Dotor J, Redrado M, Bleau A-M, Manrique I, de Aberasturi AL, et al. Co-migration of colon cancer cells and CAFs induced by TGFβ1 enhances liver metastasis. Cell Tissue Res. 2015 [cited 2019 Oct 11];359:829–39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25557989.
Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, Wever O De. Cancer-Associated Fibroblasts Connect Metastasis-Promoting Communication in Colorectal Cancer. Front Oncol. Frontiers Media SA; 2015 [cited 2019 Oct 11];5:63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25853091.
Hawinkels LJAC, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. Nature Publishing Group; 2014 [cited 2019 Oct 11];33:97–107. Available from: http://www.nature.com/articles/onc2012536.
Tommaso C, Giovanna P, Livio M, Giovanna D, Valeria R, Pamela Z, Angelo L, Gianluigi M, Sabatino Lina CV. Friend or foe?: The tumour microenvironment dilemma in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2017;1867:1–18.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98.
Derynck R, Budi EH. Specificity, versatility, and control of TGF-β family signaling. Sci signal. American Association for the Advancement of Science; 2019 [cited 2020 Apr 9];12. Available from: https://stke.sciencemag.org/content/12/570/eaav5183
Xing F, Saidou JWK. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed). 2010;1:166–79.
Berdiel-Acer M, Sanz-Pamplona R, Calon A, et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol Oncol. 2014;8:1290–305.
Parascandolo A, Bonavita R, Astaburuaga R, et al. Effect of naive and cancer-educated fibroblasts on colon cancer cell circadian growth rhythm. Cell Death Dis. 2020;11:289.
Cui L, Ohuchida K, Mizumoto K, Moriyama T, Onimaru M, Nakata K, et al. Prospectively Isolated Cancer-Associated CD10+ Fibroblasts Have Stronger Interactions with CD133+ Colon Cancer Cells than with CD133− Cancer Cells. Ng IOL, editor. PLoS One. Public library of science; 2010 [cited 2020 Nov 6];5:e12121. Available from: https://dx.plos.org/https://doi.org/10.1371/journal.pone.0012121
Funding
This work was funded by the National Science Center of Poland (grant no. 2012/07/B/NZ3/00209).
Author information
Authors and Affiliations
Contributions
M.M–K. conceived and designed the experiments; M.M.-K., B.Z., K.S.-W., and A.S.-B. performed the biological research; M.M.-K. and B.Z. performed analysis and interpreted the data; M.K.-Sz. and B.Z. critically revised the intellectual content of the manuscript; M.M.-K. wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Informed consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mizerska-Kowalska, M., Sawa-Wejksza, K., Sławińska-Brych, A. et al. Neutral endopeptidase depletion decreases colon cancer cell proliferation and TGF-β1 synthesis in indirect co-cultures with normal colon fibroblasts. Clin Transl Oncol 23, 1405–1414 (2021). https://doi.org/10.1007/s12094-020-02537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02537-x