Abstract
Background
Reduction of surgeries in axillary has been proved feasible in breast cancer with negative and limited involved axillary lymph nodes. However, for women with a heavy axillary burden, the extent of dissection is still arguable.
Patients and methods
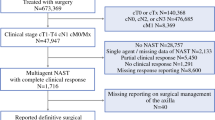
From a total of 7042 patients with breast cancer who underwent surgical treatments between 2008 and 2014, 692 (9.85%) patients with the axillary staging of N2–3M0 were classified into Level I–II dissection group and Level I–III dissection group. 203 pairs of patients were matched by the propensity score.
Results
The positive rate of level-III lymph nodes is 62.4% in patients who underwent Level I–III dissection. There are 67 (22.1%) patients who experienced rise in staging from N2 to N3 due to level-III dissection. With a median follow-up of 62.4 months, no significant difference was observed in RFS (P = 0.897), MFS (P = 0.610) and OS (P = 0.755) between level I–II group and level I–III group. The same results were observed in the independent analysis of neoadjuvant and non-neoadjuvant subgroups. The binary regression model showed the positivity of level-III is only associated with involved lymph nodes in level-II.
Conclusion
Additional level-III dissection has a limited impact on survival but still valuable in an accurate stage. The reduction of surgeries in axillary should be treated with discretion in breast cancer patients with a heavy axillary burden.


Similar content being viewed by others
References
Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 1997;15(6):2345–50. https://doi.org/10.1200/JCO.1997.15.6.2345.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–8. https://doi.org/10.1097/00000658-199409000-00015(discussion 8-401).
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–26. https://doi.org/10.1001/jama.2017.11470.
Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(10):1385–93. https://doi.org/10.1016/S1470-2045(18)30380-2.
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 1.2016. JNCCN. 2015;13(12):1475–85.
Nouso K, Miyahara K, Uchida D, Kuwaki K, Izumi N, Omata M, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br J Cancer. 2013;109(7):1904–7. https://doi.org/10.1038/bjc.2013.542.
Telli ML, Gradishar WJ, Ward JH. NCCN guidelines updates: breast cancer. JNCCN. 2019;17(5.5):552–5. https://doi.org/10.6004/jnccn.2019.5006.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)dagger. Ann Oncol: Off J Eur Soc Med cal Oncol/ESMO. 2018;29(8):1634–57. https://doi.org/10.1093/annonc/mdy192.
Orr RK. The impact of prophylactic axillary node dissection on breast cancer survival–a Bayesian meta-analysis. Ann Surg Oncol. 1999;6(1):109–16.
Wright FC, Walker J, Law CH, McCready DR. Outcomes after localized axillary node recurrence in breast cancer. Ann Surg Oncol. 2003;10(9):1054–8.
Konkin DE, Tyldesley S, Kennecke H, Speers CH, Olivotto IA, Davis N. Management and outcomes of isolated axillary node recurrence in breast cancer. Archives of surgery. 2006;141(9):867–72. https://doi.org/10.1001/archsurg.141.9.867(discussion 72-4).
Dillon MF, Advani V, Masterson C, O’Loughlin C, Quinn CM, O’Higgins N, et al. The value of level III clearance in patients with axillary and sentinel node positive breast cancer. Ann Surg. 2009;249(5):834–9. https://doi.org/10.1097/SLA.0b013e3181a40821.
Yildirim E, Berberoglu U. Lymph node ratio is more valuable than level III involvement for prediction of outcome in node-positive breast carcinoma patients. World J Surg. 2007;31(2):276–89. https://doi.org/10.1007/s00268-006-0487-5.
Kodama H, Nio Y, Iguchi C, Kan N. Ten-year follow-up results of a randomised controlled study comparing level-I vs level-III axillary lymph node dissection for primary breast cancer. Br J Cancer. 2006;95(7):811–6. https://doi.org/10.1038/sj.bjc.6603364.
Park TS, Thomas SM, Rosenberger LH, Fayanju OM, Plichta JK, Blitzblau RC, et al. The association of extent of axillary surgery and survival in women with N2-3 invasive breast cancer. Ann Surg Oncol. 2018;25(10):3019–29. https://doi.org/10.1245/s10434-018-6587-2.
Lilleborge M, Falk RS, Russnes H, Sauer T, Ursin G, Hofvind S. Risk of breast cancer by prior screening results among women participating in Breast Screen Norway. Cancer. 2019. https://doi.org/10.1002/cncr.32330.
Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in asians versus the US population. J Natl Cancer Inst. 2019. https://doi.org/10.1093/jnci/djz090.
Mori M, Fujimori M, van Vliet LM, Yamaguchi T, Shimizu C, Kinoshita T, et al. Explicit prognostic disclosure to Asian women with breast cancer: a randomized, scripted video-vignette study (J-SUPPORT1601). Cancer. 2019. https://doi.org/10.1002/cncr.32327.
Kong Y, Yang L, Tang H, Lv N, Xie X, Li J, et al. A nation-wide multicenter retrospective study of the epidemiological, pathological and clinical characteristics of breast cancer in situ in Chinese women in 1999–2008. PLoS ONE. 2013;8(11):e81055. https://doi.org/10.1371/journal.pone.0081055.
Early Breast Cancer Trialists’ Collaborative G. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. https://doi.org/10.1016/s1470-2045(17)30777-5.
Pelizzari G, Gerratana L, Basile D, Fanotto V, Bartoletti M, Liguori A, et al. Post-neoadjuvant strategies in breast cancer: from risk assessment to treatment escalation. Cancer Treat Rev. 2019;72:7–14. https://doi.org/10.1016/j.ctrv.2018.10.014.
Bonneau C, Hequet D, Estevez JP, Pouget N, Rouzier R. Impact of axillary dissection in women with invasive breast cancer who do not fit the Z0011 ACOSOG trial because of three or more metastatic sentinel lymph nodes. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2015;41(8):998–1004. https://doi.org/10.1016/j.ejso.2015.04.003.
Steenbruggen TG, Steggink LC, Seynaeve CM, van der Hoeven JJM, Hooning MJ, Jager A, et al. High-dose chemotherapy with hematopoietic stem cell transplant in patients with high-risk breast cancer and 4 or more involved axillary lymph nodes: 20-year follow-up of a phase 3 randomized clinical trial. JAMA Oncol. 2020;6(4):528–34. https://doi.org/10.1001/jamaoncol.2019.6276.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This retrospective study was approved by the Ethical Review Committee of Sun Yat-sen University Cancer Center and conducted following the principles of the Declaration of Helsinki. Nine hundred and forty-five consecutive female patients diagnosed with pathologic N2–3 (pN2–3) invasive breast cancer between October 2008 and December 2014 were identified from a total of 7042 patients with breast cancer in the Sun Yat-Sen University Cancer Center Database.
Informed consent
Patients’ informed consent was obtained before conducting the treatment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12094_2020_2444_MOESM1_ESM.tif
Figure S1—Kaplan–Meier curves for locoregional recurrence-free survival (RFS), distant metastasis-free survival (MFS) and overall survival (OS) in L-II group and L-III group before match. A, C, and E: neoadjuvant population and, B, D and F: non-adjuvant population. Supplementary material 1 (TIFF 155 kb)
12094_2020_2444_MOESM2_ESM.tif
Figure S2—Kaplan–Meier locoregional RFS curves in the subgroups of level III lymph nodes resected population. Supplementary material 2 (TIFF 1737 kb)
12094_2020_2444_MOESM3_ESM.tif
Figure S3—Kaplan–Meier distant MFS curves in the subgroups of level III lymph nodes resected population. Supplementary material 3 (TIFF 1892 kb)
12094_2020_2444_MOESM4_ESM.tif
Figure S4—Kaplan–Meier OS curves in the subgroups of level III lymph nodes resected population. Supplementary material 4 (TIFF 1788 kb)
Rights and permissions
About this article
Cite this article
Kong, Y., Yang, A., Xie, X. et al. Impact of the extent of axillary surgery in patients with N2–3 disease in the de-escalation era: a propensity score-matched study. Clin Transl Oncol 23, 526–535 (2021). https://doi.org/10.1007/s12094-020-02444-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02444-1