Abstract
Purpose
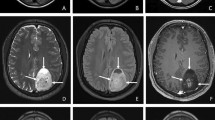
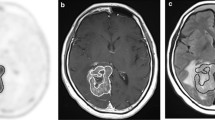
This study investigated the degree of tumor cell infiltration in the tumor cavity and ventricle wall based on fluorescent signals of 5-aminolevulinic acid (5-ALA) after removal of the magnetic resonance (MR)-enhancing area and analyzed its prognostic significance in glioblastoma.
Methods
Twenty-five newly developed isocitrate dehydrogenase (IDH)-wildtype glioblastomas with complete resection both of MR-enhancing lesions and strong purple fluorescence on resection cavity were retrospectively analyzed. The fluorescent signals of 5-ALA were divided into strong purple, vague pink, and blue colors. The pathologic findings were classified into massively infiltrating tumor cells, infiltrating tumor cells, suspicious single-cell infiltration, and normal-appearing cells. The pathological findings were analyzed according to the fluorescent signals in the resection cavity and ventricle wall.
Results
There was no correlation between fluorescent signals and infiltrating tumor cells in the resection cavity (p = 0.199) and ventricle wall (p = 0.704) after resection of the MR-enhancing lesion. The median progression-free survival (PFS) and median overall survival (OS) were 12.5 (± 2.1) and 21.1 (± 3.5) months, respectively. In univariate analysis, the presence of definitive infiltrating tumor cells in the resection cavity and ventricle wall was significantly related to the PFS (p = 0.002) and OS (p = 0.027). In multivariate analysis, the absence of definitive infiltrating tumor cells improved PFS (hazard ratio: 0.184; 95% CI: 0.049–0.690, p = 0.012) and OS (hazard ratio: 0.124; 95% CI: 0.015–0.998, p = 0.050).
Conclusions
After resection both of the MR-enhancing lesions and strong purple fluorescence on resection cavity, there was no correlation between remnant fluorescent signals and infiltrating tumor cells. The remnant definitive infiltrating tumor cells in the resection cavity and ventricle wall significantly influenced the prognosis of patients with glioblastoma. Aggressive surgical removal of infiltrating tumor cells may improve their prognosis.




Similar content being viewed by others
References
Hadjipanayis CG, Stummer W. 5-ALA and FDA approval for glioma surgery. J Neurooncol. 2019;141(3):479–86.
Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977–88.
Michael AP, Watson VL, Ryan D, Delfino KR, Bekker SV, Cozzens JW. Effects of 5-ALA dose on resection of glioblastoma. J Neurooncol. 2019;141(3):523–31.
Roh TH, Park HH, Kang SG, Moon JH, Kim EH, Hong CK, et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: A single-center analysis. Medicine (Baltimore). 2017;96(27):e7422.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42(3):518–25.
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003–13.
Haider SA, Lim S, Kalkanis SN, Lee IY. The impact of 5-aminolevulinic acid on extent of resection in newly diagnosed high grade gliomas: a systematic review and single institutional experience. J Neurooncol. 2019;141(3):507–15.
Diez Valle R, Hadjipanayis CG, Stummer W. Established and emerging uses of 5-ALA in the brain: an overview. J Neurooncol. 2019;141(3):487–94.
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401.
Della Puppa A, Munari M, Gardiman MP, Volpin F. Combined fluorescence using 5-aminolevulinic acid and fluorescein sodium at glioblastoma border: intraoperative findings and histopathologic data about three newly diagnosed consecutive cases. World Neurosurg. 2019;122:e856–e863863.
Stummer W, Suero ME. Fluorescence imaging/agents in tumor resection. Neurosurg Clin N Am. 2017;28(4):569–83.
Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74(3):310–9.
Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article J Neurosurg. 2011;114(3):595–603.
Moiyadi AV, Shetty P, Sridhar E. Periventricular glioblastomas and ependymal involvement interrogated using intraoperative fluorescence - a pathological correlative study. Br J Neurosurg. 2017;31(1):107–12.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (2017R1A1A1A05001020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone were responsible for the content and writing of the paper.
Ethical approval
This retrospective study was approved by our Institutional Review Board.
Informed consent
This manuscript doesn’t contain any individual person’s data in any form (including individual details, images or videos). The need for written informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.K., Jung, T.Y., Jung, S. et al. Relationship between tumor cell infiltration and 5-aminolevulinic acid fluorescence signals after resection of MR-enhancing lesions and its prognostic significance in glioblastoma. Clin Transl Oncol 23, 459–467 (2021). https://doi.org/10.1007/s12094-020-02438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02438-z