Abstract
Background
The incidence of breast cancer (BC) is the highest among women. Identification of miRNAs as biomarkers may help to improve the diagnosis of BC. The purpose of this study was to assess the expression levels of miR-1976 in plasma samples and the biological functions in the progression of BC.
Methods
The expression levels of miR-1976 in plasma samples and tissues were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The associations between the expression levels and clinicopathological features were studied. Cell supernatants were used to simulate circulation. The biological functions of miR-1976 were assessed in vitro and in vivo.
Results
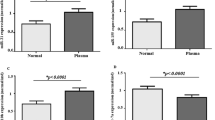
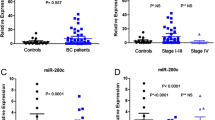
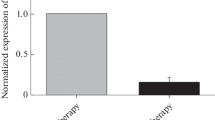
The expression levels of miR-1976 in plasma samples were found significantly lower in patients with BC than those in healthy controls, and were associated with Ki-67. The expression levels in BC tissues were lower than those in adjacent normal tissues, and were correlated with the number of lymph nodes and Ki-67. The expression levels in BC cell supernatants and cell lines were lower than that in normal human breast epithelial cell line HBL-100. miR-1976 knockdown promoted proliferation in vitro and in vivo.
Conclusion
miR-1976 may serve as a promising non-invasive biomarker for the diagnosis of BC in the future.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics. CA Cancer J Clin. 2019;69(6):438–51.
Abubakar M, Sung H, Bcr D, Guida J, Tang TS, Pfeiffer RM, Yang XR. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018;20(1):114.
Rojas K, Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016;59(4):651–72.
Tabar L, Dean PB, Chen TH, Yen AM, Chen SL, Fann JC, Chiu SY, Ku MM, Wu WY, Hsu CY, Chen YC, Beckmann K, Smith RA, Duffy SW. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer. 2019;125(4):515–23.
Guo R, Lu G, Qin B, Fei B. Ultrasound imaging technologies for breast cancer detection and management: a review. Ultrasound Med Biol. 2018;44(1):37–70.
Hill DA, Haas JS, Wellman R, Hubbard RA, Lee CI, Alford-Teaster J, Wernli KJ, Henderson LM, Stout NK, Tosteson ANA, Kerlikowske K, Onega T. Utilization of breast cancer screening with magnetic resonance imaging in community practice. J Gen Intern Med. 2018;33(3):275–83.
Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–5.
Kristiansen S, Jorgensen LM, Hansen MH, Nielsen D, Soletormos G. Concordance of hypermethylated DNA and the tumor markers CA 15-3, CEA, and TPA in serum during monitoring of patients with advanced breast cancer. Biomed Res Int. 2015;2015:986024.
Yuan Z, Baker K, Redman MW, Wang L, Adams SV, Yu M, Dickinson B, Makar K, Ulrich N, Bohm J, Wurscher M, Westerhoff M, Medwell S, Moonka R, Sinanan M, Fichera A, Vickers K, Grady WM. Dynamic plasma microRNAs are biomarkers for prognosis and early detection of recurrence in colorectal cancer. Br J Cancer. 2017;117(8):1202–10.
Hogg MC, Raoof R, El Naggar H, Monsefi N, Delanty N, O’Brien DF, Bauer S, Rosenow F, Henshall DC, Prehn JH. Elevation in plasma tRNA fragments precede seizures in human epilepsy. J Clin Invest. 2019;129(7):2946–51.
Ma G, Song G, Zou X, Shan X, Liu Q, Xia T, Zhou X, Zhu W. Circulating plasma microRNA signature for the diagnosis of cervical cancer. Cancer Biomark. 2019;26(4):491–500.
Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9(10):775–89.
Qiu Z, Guo W, Wang Q, Chen Z, Huang S, Zhao F, Yao M, Zhao Y, He X. MicroRNA-124 reduces the pentose phosphate pathway and proliferation by targeting PRPS1 and RPIA mRNAs in human colorectal cancer cells. Gastroenterology. 2015;149(6):1587–98.
Wang B, Wu H, Chai C, Lewis J, Pichiorri F, Eisenstat DD, Pomeroy SL, Leng RP. MicroRNA-1301 suppresses tumor cell migration and invasion by targeting the p53/UBE4B pathway in multiple human cancer cells. Cancer Lett. 2017;401:20–322.
He C, Wang L, Zhang J, Xu H. Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly targeting RASSF8 in gastric cancer. Mol Cancer. 2017;16(1):35.
Lu Z, He Q, Liang J, Li W, Su Q, Chen Z, Wan Q, Zhou X, Cao L, Sun J, Wu Y, Liu L, Wu X, Hou J, Lian K, Wang A. miR-31–5p is a potential circulating biomarker and therapeutic target for oral cancer. Mol Ther Nucleic Acids. 2019;16:471–80.
Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann Surg. 2014;259(4):735–43.
Chen G, Hu J, Huang Z, Yang L, Chen M. MicroRNA-1976 functions as a tumor suppressor and serves as a prognostic indicator in non-small cell lung cancer by directly targeting PLCE1. Biochem Biophys Res Commun. 2016;473(4):1144–51.
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–38.
Rodriguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, Sandvig K, Line A, Llorente A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer. 2017;16(1):156.
Qin W, Tsukasaki Y, Dasgupta S, Mukhopadhyay N, Ikebe M, Sauter ER. Exosomes in human breast milk promote EMT. Clin Cancer Res. 2016;22(17):4517–24.
Kilchert C, Wittmann S, Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Biol. 2016;17(4):227–39.
Zheng M, Hou L, Ma Y, Zhou L, Wang F, Cheng B, Wang W, Lu B, Liu P, Lu W, Lu Y. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol Cancer. 2019;18(1):76.
El-Arabey AA, Denizli M, Kanlikilicer P, Bayraktar R, Ivan C, Rashed M, Kabil N, Ozpolat B, Calin GA, Salama SA, Abd-Allah AR, Sood AK, Lopez-Berestein G. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal. 2020;68:109539.
Khoury T, Arshad A, Bogner P, Ramnath N, Zhang S, Chandrasekhar R, Wilding G, Alrawi S, Tan D. Apoptosis-related (survivin, Bcl-2), tumor suppressor gene (p53), proliferation (Ki-67), and non-receptor tyrosine kinase (Src) markers expression and correlation with clinicopathologic variables in 60 thymic neoplasms. Chest. 2009;136(1):220–8.
Lawrence NF, Hammond MR, Frederick DT, Su Y, Dias-Santagata D, Deng A, Selim MA, Mahalingam M, Flaherty KT, Hoang MP. Ki-67, p53, and p16 expression, and G691S RET polymorphism in desmoplastic melanoma (DM): a clinicopathologic analysis of predictors of outcome. J Am Acad Dermatol. 2016;75(3):595–602.
Goncalves R, Ma C, Luo J, Suman V, Ellis MJ. Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat Rev Clin Oncol. 2012;9(4):223–9.
Acknowledgements
All authors wish to thank all the patients and family members for participating in this study.
Funding
This study was funded by the Natural Science Foundation of China (81572607) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1158).
Author information
Authors and Affiliations
Contributions
SW and TX contributed to the design of the study, the interpretation of data, the revision of the article as well as final approval of the version to be submitted. JW, GM and XH performed the statistical analysis, and drafted and revised the article. JW, GM, XH, ML, and XW performed the experimental study. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no competing interests.
Ethical approval
All patients involved consented to participate under the Clinical Patient ethical statement. The study was approved by the Institutional Ethical Board of the First Affiliated Hospital of Nanjing Medical University (Number 2017-SR-171).
Informed consent
Informed consent was obtained before surgery.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Ma, G., Han, X. et al. The low expression of miR-1976 in plasma samples indicating its biological functions in the progression of breast cancer. Clin Transl Oncol 22, 2111–2120 (2020). https://doi.org/10.1007/s12094-020-02361-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02361-3