Abstract
Background
Tumor metastasis is a terrifying characteristic of cancer. Numerous studies have been conducted to overcome metastasis by targeting tumor microenvironment (TME). However, due to complexity of tumor microenvironment, it remained difficult for accurate targeting. Dwarf-lillytruf tuber monomer-13 (DT-13) possess good potential against TME.
Objective
As TME is supportive for tumor metastasis, alternatively it is a challenging for therapeutic intervention. In our present study, we explored molecular mechanism through which TME induced cell migration and how DT-13 interferes in this mechanism.
Methods
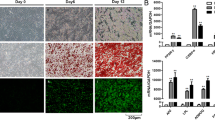
We used a novel model of co-culture system which is eventually developed in our lab. Tumor cells were co-cultured with hypoxia induced cancer-associated fibroblasts (CAF) or with chemically induced cancer-associated adipocytes (CAA). The effect of hypoxia in conditioned medium for CAF was assessed through expression of α-SMA and HIF by western blotting while oil red staining was done to assess the successful chemical induction for adipocytes (CAA), the effect of TME through conditioned medium on cell migration was analyzed by trans-well cell migration, and cell motility (wound healing) analyses. The expression changes in cellular proteins were assessed through western blotting and immunofluorescent studies.
Results and conclusion
Our results showed that tumor microenvironment has a direct role in promoting breast cancer cell migration by stromal cells; moreover, we found that DT-13 restricts this TME regulated cell migration via targeting stromal cells in vitro. Additionally we also found that DT-13 targets NMII-A for its effect on breast cancer cell migration for the regulation of stromal cells in TME.






Similar content being viewed by others
Data availability
Supporting data on our present study can be made available on request.
References
Khan GJ, et al. Understanding and responsiveness level about cervical cancer and its avoidance among young women of Pakistan. Asian Pac J Cancer Prev. 2014;15(12):4877–83.
Subramani R, et al. Role of growth hormone in breast cancer. Endocrinology. 2017;158(6):1543–55.
Hanahan D, Awada A. The hallmarks of cancer revisited. Ann Oncol. 2012;100(1):57–70.
Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450.
Wang X et al. interdependent and independent multidimensional role of tumor microenvironment on hepatocellular carcinoma. Cytokine. 2018;103:150–9.
Yang T, et al. Bile acid homeostasis paradigm and its connotation with cholestatic liver diseases. Drug Discov Today. 2019;24(1):112–28.
Michiels C, C Tellier, O Feron. Cycling hypoxia: a key feature of the tumor microenvironment. Biochim Biophys Acta (BBA) Rev Cancer. 2016; 1866(1):76–86.
Huyen CTT, et al. Chemical constituents from Cimicifuga dahurica and their anti-proliferative effects on MCF-7 breast cancer cells. Molecules. 2018;23(5):1083.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423.
Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48.
Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32(4):550–70.
Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1.
Kuzet SE, Gaggioli C. Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res. 2016;365(3):607–19.
Hugo HJ, et al. Contribution of fibroblast and mast cell (afferent) and tumor (efferent) il-6 effects within the tumor microenvironment. Cancer Microenviron. 2012;5(1):83–93.
Ghulam Jilany K, et al. Versatility of cancer associated fibroblasts: commendable targets for anti-tumor therapy. Curr Drug Targets. 2018;19(13):1573–88.
Ammirante M, et al. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci USA. 2014;111(41):14776.
Dirat B, et al. 495 Cancer-associated adipocytes exhibit an activated phenotype and contribute to early breast cancer invasion in vitro and in vivo. Eur J Cancer Suppl. 2010;8(5):126.
Nieman KM, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533.
Köhler S, Schaller V, Bausch AR. Collective dynamics of active cytoskeletal networks. PLoS ONE. 2011;6(8):e23798.
Du H, et al. DT-13 inhibits cancer cell migration by regulating NMIIA indirectly in the tumor microenvironment. Oncol Rep. 2016;36(2):721–8.
Ghulam Jilany K, et al. TGF-β1 causes emt by regulating N-acetyl glucosaminyl transferases via downregulation of non muscle myosin II-A through jnk/p38/pi3k pathway in lung cancer. Curr Cancer Drug Targets. 2017;17:1–11.
Ghulam Jilany K, et al. In-vitro pre-treatment of cancer cells with Tgf-β1: a novel approach of tail vein lung cancer metastasis mouse model for anti-metastatic studies. Curr Mol Pharmacol. 2019;12(4):249–60.
Wei XH, et al. DT-13 attenuates human lung cancer metastasis via regulating NMIIA activity under hypoxia condition. Oncol Rep. 2016;36(2):991–9.
Evenram S, et al. Myosin IIA regulates cell motility and actomyosin–microtubule crosstalk. Nat Cell Biol. 2007;9(3):299–309.
Zhang Y, et al. DT-13 suppresses MDA-MB-435 cell adhesion and invasion by inhibiting MMP-2/9 via the p38 MAPK pathway. Mol Med Rep. 2012;6(5):1121.
Sen-Sen L, et al. The saponin DT-13 inhibits gastric cancer cell migration through down-regulation of CCR5-CCL5 axis. Chin J Nat Med. 2014;12(11):833.
Khan GJ, Rizwan M, Abbas M, Naveed M, Boyang Y, Naeem MA, Khan S, Yuan S, Baig MM, Sun L. Pharmacological effects and potential therapeutic targets of DT-13. Biomed Pharmacother. 2017. https://doi.org/10.1016/j.biopha.2017.10.101.
Khan GJ, et al. Pharmacological effects and potential therapeutic targets of DT-13. Biomed Pharmacother. 2018;97:255–63.
Af S, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299(5613):1743–7.
Le CC, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88(2):489.
Dahan I, et al. The tumor suppressor Lgl1 regulates NMII-A cellular distribution and focal adhesion morphology to optimize cell migration. Mol Biol Cell. 2012;23(4):591.
Tang DD, Gerlach BD. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res. 2017;18(1):54.
Stricker J, Falzone T, Gardel ML. Mechanics of the F-actin cytoskeleton. J Biomech. 2010;43(1):9–14.
Peng X, et al. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J Cell Sci. 2010;123(4):567–77.
Small JV, et al. How do microtubules guide migrating cells? Nat Rev Mol Cell Biol. 2002;3(12):957.
Jannie KM, et al. Vinculin-dependent actin bundling regulates cell migration and traction forces. Biochem J. 2015;465(3):383–93.
Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117(20):4619.
Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6(11):827–37.
Sun L, et al. The saponin monomer of dwarf lilyturf tuber, DT-13, reduces human breast cancer cell adhesion and migration during hypoxia via regulation of tissue factor. Biol Pharm Bull. 2010;33(7):1192.
Lin SS, et al. The saponin DT-13 inhibits gastric cancer cell migration through down-regulation of CCR5-CCL5 axis. Chin J Nat Med. 2014;12(11):833.
Yu XW, et al. Synergistic combination of DT-13 and topotecan inhibits human gastric cancer via myosin IIA-induced endocytosis of EGF receptorin vitro and in vivo. Oncotarget. 2016;7(22):32990.
Li H, et al. DT-13, a saponin monomer of dwarf lilyturf tuber, induces autophagy and potentiates anti-cancer effect of nutrient deprivation. Eur J Pharmacol. 2016;781:164–72.
Raval RR, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25(13):5675.
Gordan JD, et al. HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14(6):435.
Muz B, et al. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83.
Leszczynska KB, et al. Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J Clin Invest. 2015;125(6):2385–98.
Lim H, Moon A. Inflammatory fibroblasts in cancer. Arch Pharmacal Res. 2016;39(8):1–11.
Rio MC, Dali-Youcef N, Tomasetto C. Local adipocyte cancer cell paracrine loop: can “sick fat” be more detrimental? Horm Mol Biol Clin Investig. 2015;21(1):43–56.
Zhao B, et al. Hypoxia drives the transition of human dermal fibroblasts to a myofibroblast-like phenotype via the TGF-β1/Smad3 pathway. Int J Mol Med. 2016;39(1):153–9.
Ghulam Jilany K, et al. TGF-β1 causes EMT by regulating N-Acetyl Glucosaminyl transferases via downregulation of non muscle myosin II-A through JNK/P38/PI3K pathway in lung cancer. Curr Cancer Drug Targets. 2018;18(2):209–19.
Yaoborengasser A, et al. Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol Rep. 2015;33(6):2689–94.
Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66(9):4725–33.
Jacobelli J, et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11(10):953.
Morin NA, et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205(1):195.
Tanaka C, et al. GADD34 suppresses wound healing by upregulating expression of myosin IIA. Transgenic Res. 2010;19(4):637–45.
Babbin BA, Koch S, Bachar M, Conti MA, Parkos CA, Adelstein RS, Nusrat A, Ivanov AI. Non-muscle myosin IIA differentially regulates intestinal epithelial cell restitution and matrix invasion. Am J Pathol. 2009;174(2):436.
Acknowledgements
We highly acknowledge Higher Education Commission (HEC) of Pakistan for funding our research scholar Dr. Ghulam Jilany Khan for his studies in China Pharmaceutical University. We are highly thankful to Prof. Dr. Yu Boyang, School of Chinese Material Medicine, China Pharmaceutical University, for kindly providing us the drug (DT-13) with purity > 95% for the experimental purposes.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 81601034, No. 81102853, No. 81573456 and No. 81773766).
Author information
Authors and Affiliations
Contributions
Dr. YingshengGao and Dr. Xiaohui Wei designed and performed the experimentation and figured out the findings. Dr. Ghulam Jilany Khan wrote the manuscript and designed/ arranged the data for manuscript writing and contributed equally with YingshengGao, and Xiaohui Wei; Dr. Ke-Feng Zhai arranged the data for manuscript publication and corresponded for the publication. Prof. Dr. Shengtao Yuan and Prof. Dr. Li Sun supervised the studies and guided for the experimentation theme of the study, designed the whole study plan, provided the resources for paper and are corresponding authors of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Ethical approval
No human or animals were used in this study.
Informed consent
All the authors gave their consent of participation in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Y., Khan, G.J., Wei, X. et al. DT-13 inhibits breast cancer cell migration via non-muscle myosin II-A regulation in tumor microenvironment synchronized adaptations. Clin Transl Oncol 22, 1591–1602 (2020). https://doi.org/10.1007/s12094-020-02303-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02303-z