Abstract
Background
Familial adenomatous polyposis (FAP) is an Autosomal dominant inherited disorder and a rare form of colorectal cancer (CRC) that is characterized by the development of hundreds to thousands of adenomas in the rectum and colon. Mostly, cancers develop after the advent of the polyps. It appears in both sexes evenly, and the occurrence of the disease is in the second decade of life. Mitochondrial genome mutations have been reported with a variety of Tumors, but the precise role of these mutations in the pathogenicity and tumor progression is not exactly clear. Cytochrome c oxidase subunit I (COX1) is the terminal enzyme of the mitochondrial respiratory chain. The present study aims at assessing the occurrence of mtDNA mutations in COX1 gene in FAP patients and attempts to find out the cause and effect relationship between mitochondrial mutations and tumor progression.
Methods
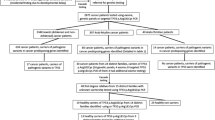
In this study, 56 FAP patients were investigated for the presence of the mutations in mitochondrial COX1 coding gene by PCR and sequencing analysis. All sequences that differed from the revised Cambridge Reference Sequence (rCRS) were classified as missense/ nonsense or silent mutations. Functional genomic studies using Bio-informatics tools were performed on the founded mutations to understand the downstream alterations in structure and function of protein.
Results
We identified 38 changes in the COX1 gene in patients with FAP symptoms. Most of them were heteroplasmic changes of missense type (25/38). Tree of the changes (G6145A, C6988A, and T7306G) were nonsense mutations and had not been reported in the literature before. Our results of bioinformatics predictions showed that the identified mutations can affect mitochondrial functions, especially if the conservative domain of the protein is concerned.
Conclusion
Our findings indicate a high frequency of mtDNA mutations in all of the FAP cases compared to matched controls. These data significantly enhance our understanding of how such mutations contribute to cancer pathologies and develop the cancer treatment methods by new diagnostic biomarkers, and new drugs for gene therapy.



Similar content being viewed by others
References
Talseth-Palmer BA. The genetic basis of colonic adenomatous polyposis syndromes. Hered Cancer Clin Pract. 2017;15:5.
Dunlop MG, Farrington SM, Bubb VJ, Cunningham C, Wright M, Curtis LJ, Butt ZA, et al. Extracolonic features of familial adenomatous polyposis in patients with sporadic colorectal cancer. Br J Cancer. 1996;74(11):1789–95.
Kerr SE, Thomas CB, Thibodeau SN, Ferber MJ, Halling KC. APC germline mutations in individuals being evaluated for familial adenomatous polyposis: a review of the Mayo Clinic experience with 1591 consecutive tests. J Mol Diagn. 2013;15(1):31–433.
Zhang Z, Liang S, Wang D, Liang S, Li Y, Wang B, Jiang T, et al. A novel pathogenic single nucleotide germline deletion in APC gene in a four generation Chinese family with familial adenomatous polyposis. Sci Rep. 2017;7(1):12357.
Plawski A, Banasiewicz T, Borun P, Kubaszewski L, Krokowicz P, Skrzypczak-Zielinska M, Lubinski J. Familial adenomatous polyposis of the colon. Hered Cancer Clin Pract. 2013;11(1):15.
Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22.
Wang D, Liang S, Zhang Z, Zhao G, Hu Y, Liang S, Zhang X, et al. A novel pathogenic splice acceptor site germline mutation in intron 14 of the APC gene in a Chinese family with familial adenomatous polyposis. Oncotarget. 2017;8(13):21327–35.
Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61(2):153–61.
Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25(34):4647–62.
Chintha R, Kaipa PR, Sekhar N, Hasan Q. Mitochondria and tumors: a new perspective. Indian J Cancer. 2013;50(3):206–13.
Salas A, Yao YG, Macaulay V, Vega A, Carracedo A, Bandelt HJ. A critical reassessment of the role of mitochondria in tumorigenesis. PLoS Med. 2005;2(11):e296.
Brandon MC, Lott MT, Nguyen KC, Spolim S, Navathe SB, Baldi P, Wallace DC. MITOMAP: a human mitochondrial genome database--2004 update. Nucleic Acids Res. 2005;33(Database issue):D611–3.
Gou H, Xue H, Yin H, Luo J, Sun X. Molecular characterization of hard ticks by cytochrome c oxidase subunit 1 sequences. Korean J Parasitol. 2018;56(6):583–8.
Rubalcava-Gracia D, Vazquez-Acevedo M, Funes S, Perez-Martinez X, Gonzalez-Halphen D. Mitochondrial versus nuclear gene expression and membrane protein assembly: the case of subunit 2 of yeast cytochrome c oxidase. Mol Biol Cell. 2018.
Kassem AM, El-Guendy N, Tantawy M, Abdelhady H, El-Ghor A, Abdel Wahab AH. Mutational hotspots in the mitochondrial D-loop region of cancerous and precancerous colorectal lesions in Egyptian patients. DNA Cell Biol. 2011;30(11):899–906.
Sui G, Zhou S, Wang J, Canto M, Lee EE, Eshleman JR, Montgomery EA, et al. Mitochondrial DNA mutations in preneoplastic lesions of the gastrointestinal tract: a biomarker for the early detection of cancer. Mol Cancer. 2006;5:73.
Zhou H, Gao M, Skolnick J. ENTPRISE-X: predicting disease-associated frameshift and nonsense mutations. PLoS ONE. 2018;13(5):e0196849.
Jakupciak JP, Maragh S, Markowitz ME, Greenberg AK, Hoque MO, Maitra A, Barker PE, et al. Performance of mitochondrial DNA mutations detecting early stage cancer. BMC Cancer. 2008;8:285.
Chinnery PF, Samuels DC, Elson J, Turnbull DM. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet. 2002;360(9342):1323–5.
Copeland WC, Wachsman JT, Johnson FM, Penta JS. Mitochondrial DNA alterations in cancer. Cancer Invest. 2002;20(4):557–69.
Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287(5460):2017–9.
Wu K, Li GC, Zhao Y, Zhang XH. Roles of mitochondria in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. Wei Sheng Yan Jiu. 2005;34(1):58–60.
Lee HC, Li SH, Lin JC, Wu CC, Yeh DC, Wei YH. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat Res. 2004;547(1–2):71–8.
Nomoto S, Yamashita K, Koshikawa K, Nakao A, Sidransky D. Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin Cancer Res. 2002;8(2):481–7.
Kirches E, Krause G, Warich-Kirches M, Weis S, Schneider T, Meyer-Puttlitz B, Mawrin C, et al. High frequency of mitochondrial DNA mutations in glioblastoma multiforme identified by direct sequence comparison to blood samples. Int J Cancer. 2001;93(4):534–8.
Maximo V, Soares P, Lima J, Cameselle-Teijeiro J, Sobrinho-Simoes M. Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: a study with emphasis on Hurthle cell tumors. Am J Pathol. 2002;160(5):1857–65.
Yeh JJ, Lunetta KL, van Orsouw NJ, Moore FD Jr, Mutter GL, Vijg J, Dahia PL, et al. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumours. Oncogene. 2000;19(16):2060–6.
Liu VW, Shi HH, Cheung AN, Chiu PM, Leung TW, Nagley P, Wong LC, et al. High incidence of somatic mitochondrial DNA mutations in human ovarian carcinomas. Cancer Res. 2001;61(16):5998–6001.
Parrella P, Xiao Y, Fliss M, Sanchez-Cespedes M, Mazzarelli P, Rinaldi M, Nicol T, et al. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 2001;61(20):7623–6.
Chen JZ, Gokden N, Greene GF, Mukunyadzi P, Kadlubar FF. Extensive somatic mitochondrial mutations in primary prostate cancer using laser capture microdissection. Cancer Res. 2002;62(22):6470–4.
Chen JZ, Gokden N, Greene GF, Green B, Kadlubar FF. Simultaneous generation of multiple mitochondrial DNA mutations in human prostate tumors suggests mitochondrial hyper-mutagenesis. Carcinogenesis. 2003;24(9):1481–7.
Jeronimo C, Nomoto S, Caballero OL, Usadel H, Henrique R, Varzim G, Oliveira J, et al. Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene. 2001;20(37):5195–8.
Canter JA, Kallianpur AR, Parl FF, Millikan RC. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African–American women. Cancer Res. 2005;65(17):8028–33.
Liu VW, Yang HJ, Wang Y, Tsang PC, Cheung AN, Chiu PM, Ng TY, et al. High frequency of mitochondrial genome instability in human endometrial carcinomas. Br J Cancer. 2003;89(4):697–701.
Herrmann PC, Gillespie JW, Charboneau L, Bichsel VE, Paweletz CP, Calvert VS, Kohn EC, et al. Mitochondrial proteome: altered cytochrome c oxidase subunit levels in prostate cancer. Proteomics. 2003;3(9):1801–10.
Krieg RC, Knuechel R, Schiffmann E, Liotta LA, Petricoin EF 3rd, Herrmann PC. Mitochondrial proteome: cancer-altered metabolism associated with cytochrome c oxidase subunit level variation. Proteomics. 2004;4(9):2789–95.
Ray AM, Zuhlke KA, Levin AM, Douglas JA, Cooney KA. Petros JA Sequence variation in the mitochondrial gene cytochrome c oxidase subunit I and prostate cancer in African American men. Prostate. 2009;69(9):956–60.
Mishmar D, Ruiz-Pesini E, Golik P, Macaulay V, Clark AG, Hosseini S, Brandon M, et al. Natural selection shaped regional mtDNA variation in humans. Proc Natl Acad Sci U S A. 2003;100(1):171–6.
Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303(5655):223–6.
Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005;102(3):719–24.
Herrero-Martin MD, Pineda M, Briones P, Lopez-Gallardo E, Carreras M, Benac M, Angel Idoate M, et al. A new pathologic mitochondrial DNA mutation in the cytochrome oxidase subunit I (MT-CO1). Hum Mutat. 2008;29(8):E112–E122.
Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14.
Park SY, Shin MG, Kim HR, Oh JY, Kim SH, Shin JH, Cho YB, et al. Alteration of mitochondrial DNA sequence and copy number in nasal polyp tissue. Mitochondrion. 2009;9(5):318–25.
Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Biol. 2013;5(11):a021220.
Scott TA, Arnold R, Petros JA. Mitochondrial cytochrome c oxidase subunit 1 sequence variation in prostate cancer. Scientifica (Cairo). 2012;2012:701810.
Tanaka M, Takeyasu T, Fuku N, Li-Jun G, Kurata M. Mitochondrial genome single nucleotide polymorphisms and their phenotypes in the Japanese. Ann N Y Acad Sci. 2004;1011:7–20.
Dennerlein S, Rehling P. Human mitochondrial COX1 assembly into cytochrome c oxidase at a glance. J Cell Sci. 2015;128(5):833–7.
Acknowledgements
We thank all the patients for providing blood samples for scientific research. The study was funded and approved by Yazd University Human Research Committee.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed toward data analysis, drafting the initial manuscript, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All research was carried out in compliance with the Helsinki Declaration. This study was approved by the Ethics Committee of the Yazd University board.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afkhami, E., Heidari, M.M., Khatami, M. et al. Detection of novel mitochondrial mutations in cytochrome C oxidase subunit 1 (COX1) in patients with familial adenomatous polyposis (FAP). Clin Transl Oncol 22, 908–918 (2020). https://doi.org/10.1007/s12094-019-02208-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02208-6