Abstract
Introduction
Low-molecular-weight heparin (LMWH) is the standard treatment for cancer-associated venous thromboembolism (VTE). There have been no specific studies evaluating bemiparin for VTE in people with cancer. The aim of this study is to evaluate the effects of bemiparin for long-term treatment of VTE in routine clinical practice.
Methods/patients
Prospective observational study. Consecutive patients with active cancer and VTE, under treatment with bemiparin for at least 6 months, were recruited.
Results
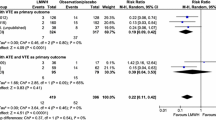
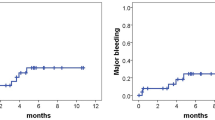
We included 89 patients. The 6- and 9-month cumulative VTE recurrence rates were 2.4% and 5.9%, respectively. The 6-month cumulative rate of major bleeding was 1.3%, and of clinically relevant non-major bleeding, 8%.
Conclusions
The incidence of events in this study is lower than that reported in randomized trials. Bemiparin is effective and safe for the long-term treatment of cancer-associated VTE in routine clinical practice.

Similar content being viewed by others
References:s
Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arc Intern Med 2006;166:458–64.
Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007;15:110.
Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632–634.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52.
Lyman GH, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract 2015;11:e442–e444.
Farge D, Debourdeau P, Beckers M, Baglin C, Bauersachs RM, Brenner B, Brilhante D, Falanga A, Gerotzafias GT. Haim N et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost 2013;11:56–70.
Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, Le Maignan C, Extra JM, Cottu P, Farge D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch Intern Med. 2002;162:1729–35.
Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J; Investigators O. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Haemost 2006;12:389–396.
Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153.
Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R, Wong T, Cook R, Solymoss S, Poon MC, et al. Long-term-low-molecular-weight heparin versus usual care in proximal vein thrombosis patients with cancer. Am J Med. 2006;119:1062–72.
Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA, Investigators C. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA. 2015;314:677–86.
Kakkar VV, Gebska M, Kadziola Z, Saba N, Carrasco P. Bemiparin Invesigators. Low-molecular-weight heparin in the acute and long-term treatment of deep vein thrombosis. Thromb Haemost 2003;89:674–80.
Kakkar VV, Balibrea JL, Martínez-González J, Prandoni P, CANBESURE Study Group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223–29.
Schulman S, Kearon C; Subcommittee on control of anticoagulation of the Scientific and Standardization Committee of International Society on thrombosis and haemostasis. Definition of major bleeding in clinical investigations of antihemostatis medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694.
Austin PC, Fine Jason P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–400.
Prandoni P, Lensing AWA, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484–88
Hyers TM, Agnelli G, Hull RD et al. Antithrombotic therapy for venous thromboembolic disease. Chest 2001;119(1 Suppl):176S–193S
Raskob GE, van ESN, Verhamme P, et al. Hokusai VTE cancer investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624
Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J. Clin Oncol. 2018;36:2017–23.
Streiff MB, Holmstrom B, Angelini D, Ashrani A, et al. National comprehensive cancer network clinical practice guidelines in oncology: cancer associated venous thromboembolic disease-vesion 2. 2018. J Natl Compr Cancer Netw. 2018;16(11):1289–1303.
Funding
This project was funded in partly by a restricted educational grant from Laboratorios Farmacéuticos Rovi, SA. Second International Competition for grants for biomedical research bemiparin (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
The work has been conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients signed the informed consent form before enrolling in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pina, E., Antonio, M., Peris, J. et al. Bemiparin as a long-term treatment for venous thrombosis in cancer patients: the ELEBAMA study. Clin Transl Oncol 22, 616–620 (2020). https://doi.org/10.1007/s12094-019-02159-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02159-y