Abstract
Objectives
In recent years, docetaxel, cisplatin and fluorouracil (TPF)-based induction chemotherapy (IC) has been widely applied in the treatment of locoregionally advanced nasopharyngeal carcinoma (LA-NPC). However, it remains unclear whether TPF is the ideal IC regimen. Thus, we carried out a meta-analysis to compare the efficacy and safety of TPF-based IC plus concurrent chemoradiotherapy (CCRT) versus CCRT alone or double-drug-based IC plus CCRT for LA-NPC.
Methods
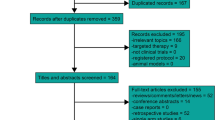
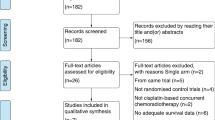
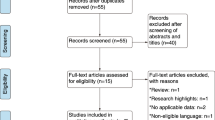
We systematically searched PubMed, Embase and the Cochrane Library from inception until December 2018. After rigorous screening of all relevant studies that reported the use of TPF-based IC followed by CCRT for patients with LA-NPC, eight studies met the inclusion criteria and were assessed for design and quality. Among them, three articles were classified as having a high risk of bias and were excluded from the meta-analysis. The outcomes, including overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), locoregional failure-free survival (LRFFS) and incidence of adverse events, were pooled with the use of hazard ratio (HR) or odds ratio (OR). Heterogeneity and sensitivity analyses were also carried out.
Results
Five trials involving 4223 patients were included in the meta-analysis. Compared to CCRT alone, TPF-based IC plus CCRT significantly improved OS (HR 0.54, 95% confidence interval [CI] 0.35–0.84, P = 0.006), PFS (HR 0.64, 95% CI 0.46–0.88, P = 0.006), LRFFS (HR 0.57, 95% CI 0.34–0.94, P = 0.03), and DMFS (HR 0.58, 95% CI 0.38–0.88, P = 0.01). Moreover, compared to double-drug-based IC plus CCRT, OS (HR 0.74, 95% CI 0.62–0.87, P = 0.0004), PFS (HR 0.76, 95% CI 0.66–0.88, P = 0.0001) and LRFFS (HR 0.75, 95% CI 0.61–0.92, P = 0.006) were also significantly improved by TPF-based IC plus CCRT. Notably, TPF-based IC plus CCRT mainly led to an increased risk of hematologic toxicities, such as leucopenia (OR = 3.20, 95% CI 2.13–4.81, P < 0.0001) and neutropenia (OR = 3.84, 95% CI 0.66–22.36, P = 0.13). However, these were uncomplicated and manageable with growth factor support.
Conclusions
Compared to CCRT alone or double-drug-based IC plus CCRT, TPF-based IC plus CCRT results in better survival outcomes with manageable toxicities. Thus, it is reasonable to recommend the addition of TPF-based IC to CCRT as an excellent choice for patients with LA-NPC.





Similar content being viewed by others
References
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–24.
Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):326–1334.
Lee AWM, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233–44.
Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–7.
Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2010;80(3):661–8.
Wu F, Wang R, Lu H, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112(1):106–11.
Li WF, Chen L, Sun Y, Ma J. Induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2016;35(1):94.
Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55.
Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23.
Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–20.
Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–9.
Kong L, Zhang Y, Hu C, et al. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of 2 parallel phase 2 clinical trials. Cancer. 2017;123(12):2258–67.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology for head and neck cancer, Version 2. 2017. http://www.nccn.org.
Chan ATC, Gregoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;23(suppl 7):vii83–vii85.
Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64(1):47–56.
Vermorken JB, Remenar E, Gorlia T, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–704.
Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–15.
Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264.
Higgins JPT, et al. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. 2011. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org.
Gu WJ, Wang F, Tang L, Liu JC. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled and observational studies. Chest. 2015;147(2):335–46.
Yang Y, Zhang D, Feng N, et al. Increased intake of vegetables, but not fruit, reduce risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147(5):1031–42.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Smith GD, Schneider M, Minder C. Egger-Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Zeng Z, Yan RN, Tu L, et al. Assessment of concurrent chemoradiotherapy plus induction chemotherapy in advanced nasopharyngeal carcinoma: cisplatin, fluorouracil, and docetaxel versus gemcitabine and cisplatin. Sci Rep. 2018;8(1):15581.
Peng H, Tang LL, Chen BB, et al. Optimizing the induction chemotherapy regimen for patients with locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2018;79:40–6.
Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006–02). Ann Oncol. 2018;29(3):731–6.
Liu GY, Lv X, Wu YS, et al. Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. Cancer Commun. 2018;38(1):21.
Mnejja W, Toumi N, Fourati N, et al. Neoadjuvant chemotherapy with concurrent chemoradiotherapy in the treatment of nasopharyngeal cancer: Southern Tunisian experience. Bull Cancer. 2018;105(5):450–7.
Ou D, Blanchard P, ElKhoury C, et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;62:114–21.
Kertmen N, Aksoy S, Cengiz M, et al. Comparison of three different induction regimens for nasopharyngeal cancer. Asian Pac J Cancer Prev. 2015;16(1):59–63.
Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;20(5):505–10.
Lee AWM, Ngan RKC, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121(8):1328–38.
Liang ZG, Zhu XD, Tan AH, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy with or without adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: meta-analysis of 1,096 patients from 11 randomized controlled trials. Asian Pac J Cancer Prev. 2013;14(1):515–21.
Song Y, Wang W, Tao G, Zhou X. Survival benefit of induction chemotherapy in treatment for locally advanced nasopharyngeal carcinoma—A time-to-event meta-analysis. Oral Oncol. 2015;51(8):764–9.
Chen YP, Guo R, Lin N, et al. Efficacy of the additional neoadjuvant chemotherapy to concurrent chemoradiotherapy for patients with locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis of randomized controlled trials. J Cancer. 2015;6(9):883–92.
Yan M, Kumachev A, Siu LL, et al. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis. Eur J Cancer. 2015;51(12):1570–9.
Wang MM, Tian H, Li G, et al. Significant benefits of adding neoadjuvant chemotherapy before concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Oncotarget. 2016;7(30):48375–90.
Zheng W, Qiu S, Huang L, Pan J. Is gemcitabine and cisplatin induction chemotherapy superior in locoregionally advanced nasopharyngeal carcinoma? Pak J Med Sci. 2015;31(4):781–6.
Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. The Lancet. 2016;388(10054):1883–992.
Wang FZ, Sun QQ, Jiang C, et al. Gemcitabine/cisplatin induction chemotherapy before concurrent chemotherapy and intensity-modulated radiotherapy improves outcomes for locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8(57):96798–808.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (Grant no. 81803569) without any involvement in writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that there are no conflicts of interest regarding the manuscript.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For the type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, R., Zhu, J., Chen, X. et al. The efficacy and safety of docetaxel, cisplatin and fluorouracil (TPF)-based induction chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis. Clin Transl Oncol 22, 429–439 (2020). https://doi.org/10.1007/s12094-019-02142-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02142-7