Abstract
Background
Sarcopenia is related to poor prognosis and drug toxicities in solid tumors. The aim of our study is to investigate the predisposition of patients with metastatic colorectal carcinoma who started regorafenib treatment to sarcopenia and prolonged survival.
Methods
Patients with metastatic colorectal carcinoma who receives regorafenib were search retrospectively. Dose-limiting toxicity was defined as dose reduction or toxicity requiring drug withdrawal. Sarcopenia evaluation was made with computed tomography performed within a month before treatment. Progression-free survival and overall survival were estimated.
Results
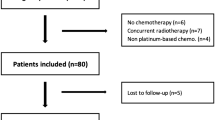
Thirty-six patients were found as suitable for the study. 63.9% of patients were found as basally sarcopenic. Dose-limiting toxicity occured 13 of 23 patients (56.5%) with basal sarcopenia, whereas only 1 of 13 patients (7.6%) with no sarcopenia exhibited dose-limiting toxicity (p = 0.005). Three patients suffered from grade 3–4 toxicity. Hand–foot syndrome, hypertension, and mucosal rash were the most seen side effects. Mean regorafenib treatment duration was 3.36 months. There was no significant difference in the progression-free survival (PFS) and the overall survival (OS) between sarcopenic patients and patients with no sarcopenia. Durations were as OS 24.2 weeks in patients with sarcopenia (95% CI 16.7–31.7), 28.1 weeks in patients with no sarcopenia (95% CI 20.5–35.7) (p = 0.36), and as PFS 14.2 weeks in patients with sarcopenia (95% CI 12.1–16.4), 14.8 weeks in patients with no sarcopenia (95% CI 9.7–20.1) (p = 0.65).
Conclusion
Dose-limiting toxicity was significantly higher in basally sarcopenic patients who were started regorafenib as treatment of metastatic colorectal carcinoma. There was no significant relationship between overall survival and progression-free survival with sarcopenia.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin. 2018;68(1):7–30.
Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD. Based on November 2016 SEER data submission, posted to the SEER web site. 2017.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Petrioli R, Chirra M, Messuti L, Fiaschi AI, Savelli V, et al. Efficacy and safety of regorafenib with 2/1 schedule for patients ≥ 75 years with metastatic colorectal cancer (mCRC) after failure of 2 lines of chemotherapy. Clin Colorectal Cancer. 2018;17(4):307–12.
Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J Urol. 2016;195(1):26–32.
Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, Ropert S, Delongchamps NB, Zerbib M, Goldwasser F. Sarcopenia and body mass index predict sunitinib induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013;108(5):1034–41.
Antonelli G, Gigante E, Iavarone M, Begini P, Sangiovanni A, Lanlicelli E, et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Europ Gastroenterol J. 2018;6(7):1039–48.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–22.
Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Annals of oncology. 2010;21(8):1594–8.
Chang SF, Lin PL. Systematic literature review and meta-analysis of the association of sarcopenia with mortality. Worldviews Evid Based Nurs. 2016;13(2):153–62.
Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver cancer. 2018;7(1):90–103.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Europ J Cancer. 2016;57:58–67.
Park SE, Hwang IG, Choi CH, Kang H, Kim BG, et al. Sarcopenia is poor prognostic factor in older patients with locally advanced rectal cancer who received preoperative or postoperative chemoradiotherapy. Medicine (Baltimore). 2018;97(48):e13363.
Kiss N, Beraldo J, Everitt S. Early skeletal muscle loss in non-small cell lung cancer patients receiving chemoradiation and relationship to survival. Support Care Cancer. 2018;26:1–8.
Funding
There is no financial support in this study
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gökyer, A., Küçükarda, A., Köstek, O. et al. Relation between sarcopenia and dose-limiting toxicity in patients with metastatic colorectal cancer who received regorafenib. Clin Transl Oncol 21, 1518–1523 (2019). https://doi.org/10.1007/s12094-019-02080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02080-4