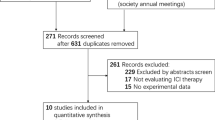
Abstract
Introduction
Many methods used to assess the effectiveness of immune checkpoint (programmed death-ligand 1 or cytotoxic T-lymphocyte-associated protein 4) inhibitors for non-small cell lung cancer (NSCLC) are insufficient, as the therapeutic benefit of these agents is often underestimated. Consequently, immune-related evaluation criteria have been developed to better reflect their efficacy. The aim of this consensus was to obtain the opinion of lung cancer experts on the adequacy of immune-response criteria for evaluating the efficacy of these treatments.
Methods
Through two rounds of a modified Delphi consensus, 18 Spanish lung cancer experts participated in a 15-item questionnaire regarding the use of immunotherapies for NSCLC and the assessment criteria used to evaluate their effectiveness.
Results
Consensus was achieved on 80% of the items in the questionnaire. The panelists agreed that although the Response Evaluation Criteria in Solid Tumors (RECIST) are standard for the evaluation of solid tumors, immune-related response criteria would be useful for measuring the efficacy of immunotherapy. In addition, they considered that an overall survival (OS) rate at 2–5 years is the most useful end point for assessing the benefit of immunotherapy, as clinical benefit may extend beyond the RECIST criteria-defined progression of disease.
Conclusions
Although immune-related response criteria have been developed to better evaluate the efficacy of immunotherapy, their use has not been validated and is restricted to investigational applications. However, they may prove to be a useful tool for measuring the efficacy of immunotherapy agents in NSCLC, especially the OS rate at 2–5 years.


Similar content being viewed by others
Abbreviations
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
References
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. https://doi.org/10.1056/NEJMoa011954.
Haslam S, Chrisp P. Bevacizumab: the evidence for its clinical potential in the treatment of nonsmall cell lung cancer. Core Evid. 2007;2(1):31–49.
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34. https://doi.org/10.1200/JCO.2007.14.5466.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. https://doi.org/10.1056/NEJMoa061884.
Socinski MA, Saleh MN, Trent DF, Dobbs TW, Zehngebot LM, Levine MA, et al. A randomized, phase II trial of two dose schedules of carboplatin/paclitaxel/cetuximab in stage IIIB/IV non-small-cell lung cancer (NSCLC). Ann Oncol. 2009;20(6):1068–73. https://doi.org/10.1093/annonc/mdn745.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–54. https://doi.org/10.1200/JCO.2011.38.4032.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. https://doi.org/10.1056/NEJMoa1504627.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. https://doi.org/10.1056/NEJMoa1501824.
Herbst RS, Baas P, Pérez-Gracia JL, Felip E, Kim DW, Han JY, et al. PD1.06 (also presented as P2.41): pembrolizumab versus docetaxel for previously treated NSCLC (KEYNOTE-010): archival versus new tumor samples for PD-L1 assessment. J Thorac Oncol. 2016;11(10S):S174–5. https://doi.org/10.1016/j.jtho.2016.08.014.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. https://doi.org/10.1016/S0140-6736(16)00587-0.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. https://doi.org/10.1038/nrc3239.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. https://doi.org/10.1158/1078-0432.CCR-09-1624.
Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198(4):737–45. https://doi.org/10.2214/AJR.11.7483.
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–43. https://doi.org/10.1158/1078-0432.CCR-13-0895.
Hoos A, Britten C. The immuno-oncology framework: enabling a new era of cancer therapy. Oncoimmunology. 2012;1(3):334–9. https://doi.org/10.4161/onci.19268.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–9. https://doi.org/10.1200/JCO.2006.07.3049.
Lee HY, Lee KS, Hwang HS, Lee JW, Ahn MJ, Park K, et al. Molecularly targeted therapy using bevacizumab for non-small cell lung cancer: a pilot study for the new CT response criteria. Korean J Radiol. 2010;11(6):618–26. https://doi.org/10.3348/kjr.2010.11.6.618.
Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009;27(3):404–10. https://doi.org/10.1200/JCO.2008.16.2545.
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–7. https://doi.org/10.1200/jco.2015.64.0391.
Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–80.
Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lázaro P, et al. The RAND/UCLA appropriateness method user’s manual. http://www.rand.org/pubs/monograph_reports/MR1269.html. Accessed 5 July 2017.
Dolgin E. Cancer immunology community seeks better end points. Nat Rev Drug Discov. 2016;15(12):807–9. https://doi.org/10.1038/nrd.2016.254.
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–97. https://doi.org/10.1093/jnci/djq310.
Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–22. https://doi.org/10.1200/JCO.2012.44.6112.
Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Janne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. https://doi.org/10.1186/s40425-016-0193-2.
Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. https://doi.org/10.1093/annonc/mds213.
Spiegel ML, editor. A phase I study of exemestane with carboplatin and pemetrexed in postmenopausal women with metastatic, non-squamous non-small cell lung cancer (Poster P.3.01). In:16th World conference on lung cancer; 2015 September 6–9; Denver, Colorado.
Mazieres J, Fehrenbacher L, Rittmeyer A, Spira AI, Park K, Smith DA, Artal-Cortes A, Lewanski CR, Braiteh FS, Yi J, He P, Kowanetz M, Waterkamp D, Ballinger M, Chen DS, Sandler A, Vansteenkiste JF. Non-classical response measured by immune-modified RECIST and post-progression treatment effects of atezolizumab in 2L/3L NSCLC: results from the randomized phase II study POPLAR. J Clin Oncol. 2016;34(15_suppl):9032.
Taylor M, Dutcus CE, Schmidt E, Bagulho T, Li D, Shumaker R, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients with selected solid tumors. Ann Oncol. 2016;27(6):266–95.
Kim HK, Heo MH, Lee HS, Sun JM, Lee SH, Ahn JS, et al. Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother Pharmacol. 2017;80(3):591–8. https://doi.org/10.1007/s00280-017-3396-4.
Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. https://doi.org/10.1016/j.ejca.2017.10.017.
Petrelli F, Coinu A, Cabiddu M, Borgonovo K, Ghilardi M, Lonati V, et al. Early analysis of surrogate endpoints for metastatic melanoma in immune checkpoint inhibitor trials. Medicine (Baltimore). 2016;95(26):e3997. https://doi.org/10.1097/MD.0000000000003997.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. https://doi.org/10.1056/NEJMoa1414428.
Acknowledgements
The authors would like to thank all panelists that participated in this project: Joaquim Bosch (Instituto Catalán de Oncología – Hospital Dr.Trueta, Girona), Manuel Cobo (Hospital Universitario Carlos Haya, Málaga), Juan Coves (Hospital Son Llatzer, Palma de Mallorca), Ramón de las Peñas (Hospital Provincial de Castellón, Castellón), Manuel Dómine (Fundación Jiménez Díaz, Madrid), Enriqueta Felip (Hospital Universitario Vall D’Hebron, Barcelona), Rosario García Campelo (Hospital Teresa Herrera, A Coruña), Javier Garde (Hospital Arnau de Vilanova de Valencia, Valencia), José Luis González-Larriba (Hospital Clínico San Carlos, Madrid), Mónica Guillot (Hospital Universitario Son Espases, Palma de Mallorca), María Guirado (Hospital General Universitario de Elche, Alicante), Amelia Insa (Hospital Clínico Universitario de Valencia, Valencia), Dolores Isla (Hospital Clínico Universitario Lozano Blesa, Zaragoza), Guillermo López-Vivanco (Hospital de Cruces Baracaldo, Vizcaya), Bartomeu Massutí (Hospital General Universitario de Alicante, Alicante), Ana Laura Ortega (Hospital Ciudad de Jaen, Jaén), Luis Paz-Ares (Hospital Universitario 12 de Octubre, Madrid), Delvys Rodríguez (Hospital Universitario Insular de Gran Canaria, Gran Canaria). Also, the authors thank Dr. Fernando Sánchez Barbero who provided assistance in the preparation of this manuscript on behalf of Springer Healthcare, and Sarah Greig, PhD, of Springer Healthcare Communications, for editing the manuscript before submission. This medical writing assistance was funded by Bristol-Myers Squibb.
Funding
Bristol-Myers Squibb promoted and financed this study without participating in its design, analysis of data, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M Provencio has served as consultant or advisor for Bristol-Myers Squibb, Celgene, Merck Sharp and Dohme, Pierre Fabre, and Roche. E. Carcereny has served as consultant or advisor for Bristol-Myers Squibb, Merck, Pfizer, and Roche; and has received travel and expenses funding from Bristol-Myers Squibb, and Pfizer. Á. Artal has participated on the speaker´s bureau and advisory boards for Bristol-Myers Squibb, Merck Sharp and Dohme, and Roche-Genentech; and has received travel and expenses funding form Bristol-Myers Squibb and Roche.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human participants performed by any of the authors, so informed consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Provencio, M., Carcereny, E. & Artal, Á. Consensus on the use of immune-related response criteria to evaluate the efficacy of immunotherapy in non-small cell lung cancer. Clin Transl Oncol 21, 1464–1471 (2019). https://doi.org/10.1007/s12094-019-02072-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02072-4