Abstract
Purpose
The standard treatment for patients with stage III non-small cell lung cancer (NSCLC), unsuitable for resection and with good performance, is definitive radiotherapy with cisplatin-based chemotherapy. Our aim is to evaluate the effect of the maximum value of standardized uptake values (SUVmax) of the primary tumor in positron emission tomography-computed tomography (PET/CT) before treatment on complete response (CR) and overall survival.
Methods
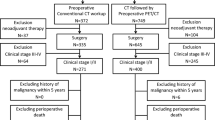
The data of 73 stage III NSCLC patients treated with concurrent definitive chemoradiotherapy (CRT) between 2008 and 2017 and had PET/CT staging in the pretreatment period were evaluated. ROC curve analysis was performed to determine the ideal cut-off value of pretreatment SUVmax to predict CR.
Results
Median age was 58 years (range 27–83 years) and 66 patients were male (90.4%). Median follow-up time was 18 months (range 3–98 months); median survival was 23 months. 1-year overall survival (OS) rate and 5-year OS rate were 72 and 19%, respectively. Median progression-free survival (PFS) was 9 months; 1-year PFS rate and 5-year PFS rate were 38 and 19%, respectively. The ideal cut-off value of pretreatment SUVmax that predicted the complete response of CRT was 12 in the ROC analysis [AUC 0.699 (0.550–0.833)/P < 0.01] with a sensitivity of 83%, and specificity of 55%. In patients with SUVmax < 12, CR rate was 60%, while, in patients with SUV ≥ 12, it was only 19% (P = 0.002). Median OS was 26 months in patients with pretreatment SUVmax < 12, and 21 months in patients with SUVmax ≥ 12 (HR = 2.93; 95% CI 17.24–28.75; P = 0.087). CR rate of the whole patient population was 26%, and it was the only factor that showed a significant benefit on survival in both univariate and multivariate analyses.
Conclusion
Pretreatment SUVmax of the primary tumor in PET/CT may predict CR in stage III NSCLC patients who were treated with definitive CRT. Having clinical CR is the only positive predictive factor for prolonged survival.


Similar content being viewed by others
References
Brambilla E, Travis WD. Lung cancer. In: Stewart BW, Wild CP, editors. World cancer report. Lyon: World Health Organization; 2014.
Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23(3175):85.
Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;2(8):2181–90.
Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8:1–20.
Ahn JS, Ahn YC, Kim JH, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:26606.
MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol. 2009;91(1):85–94.
Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG–PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3(1):6–12.
Machtay M, Duan F, Siegel BA, et al. Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol. 2013;31(30):3823–30.
Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non–small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42(2):241–51.
Ohri N, Duan F, MacHtay M, Gorelick JJ, Snyder BS, Alavi A, et al. Pretreatment FDG-PET metrics in stage III non-small cell lung cancer: ACRIN 6668/RTOG 0235. J Natl Cancer Inst. 2015;107(4):djv004.
Fried DV, Mawlawi O, Zhang L, Fave X, Zhou S, Ibbott G, et al. Stage III non-small cell lung cancer: prognostic value of FDG PET quantitative imaging features combined with clinical prognostic factors. Radiology. 2016;278(1):214–22.
van Elmpt W, Ollers M, Dingemans A-MC, Lambin P, De Ruysscher D. Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J Nucl Med. 2012;53(10):1514–20.
Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-d-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(33):8362–70.
Decoster L, Schallier D, Everaert H, Nieboer K, Meysman M, Neyns B, et al. Complete metabolic tumour response, assessed by 18-fluorodeoxyglucose positron emission tomography (18FDG-PET), after induction chemotherapy predicts a favourable outcome in patients with locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2008;62:55–61.
MacManus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92.
Ohri N, Piperdi B, Garg MK, Bodner WR, Gucalp R, Perez-Soler R, et al. Pre-treatment FDG–PET predicts the site of in-field progression following concurrent chemoradiotherapy for stage III non-small cell lung cancer. Lung Cancer. 2015;87(1):23–7.
Na F, Wang J, Li C, Deng L, Xue J, Lu Y, et al. Primary tumor standardized uptake value measured on F18-Fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol. 2014;9(6):834–42.
Zhang HQ, Yu JM, Meng X, Yue JB, Feng R, Ma L. Prognostic value of (18)F-fluorodeoxyglucose uptake in patients with non-small cell lung cancer treated by concurrent chemoradiotherapy. Zhonghua Zhong Liu Za Zhi. 2010;32(8):603–6.
Lopez Guerra JL, Gladish G, Komaki R, Gomez D, Zhuang Y, Liao Z. Large decreases in standardized uptake values after definitive radiation are associated with better survival of patients with locally advanced non-small cell lung cancer. J Nucl Med. 2012;53(2):225–33.
Paesmans M, Berghmans T, Dusart M, Garcia C, Hossein-Foucher C, Lafitte JJ, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European lung cancer working party for the international association for the study of lung cancer staging project. J Thorac Oncol. 2010;5(5):612–9.
Paesmans M, Garcia C, Wong C-YO, Patz EF, Komaki R, Eschmann S, et al. Primary tumour standardised uptake value is prognostic in nonsmall cell lung cancer: a multivariate pooled analysis of individual data. Eur Respir J. 2015;46(6):1751–61.
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG–PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC). J Thorac Oncol. 2008;3:6–12.
Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, et al. Is standardised (18)F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging. 2006;33(3):263–9.
Okereke C, Gangadharan SP, Kent MS, Nicotera SP, Shen C, DeCamp MM. Standard uptake value predicts survival in non-small cell lung cancer. Ann Thorac Surg. 2009;88(3):911–6.
Goodgame B, Pillot GA, Yang Z, Shriki J, Meyers BF, Zoole J, et al. Prognostic value of preoperative positron emission tomography in resected stage I nonsmall cell lung cancer. J Thorac Oncol. 2008;3(2):130–4.
Nair VS, Krupitskaya Y, Gould MK. Positron emission tomography 18F-fluorodeoxyglucose uptake and prognosis in patients with surgically treated, stage I non-small cell lung cancer: a systematic review. J Thorac Oncol. 2009;4(12):1473–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Research involving human participants and/or animals
Not applicable, since the study was retrospective.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Ethics/institutional review board approval of research
Faculty of Medicine, Marmara University, Istanbul, Turkey. Number: 09.2018.281 Date: 07.05.2018.
Rights and permissions
About this article
Cite this article
Ercelep, O., Alan, O., Sahin, D. et al. Effect of PET/CT standardized uptake values on complete response to treatment before definitive chemoradiotherapy in stage III non-small cell lung cancer. Clin Transl Oncol 21, 499–504 (2019). https://doi.org/10.1007/s12094-018-1949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1949-6