Abstract
Background
Everolimus with exemestane has shown promising activity in patients with hormone-receptor (HR)-positive HER2-negative endocrine-resistant advanced breast cancer. It is necessary, therefore, to characterize the safety profile of this new combination in the real-world clinical setting and in the broadest possible population.
Patients and methods
Post-menopausal women with HR-positive HER2-negative advanced breast cancer progressing after prior non-steroidal aromatase inhibitors (NSAIs) were included. The objectives of this analysis were to evaluate the safety profile of this combination in a subset of Spanish patients in the BALLET trial and to characterize grade 3 and 4 adverse events (AEs) in routine clinical practice in Spain.
Results
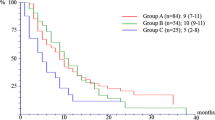
Between September 2012 and July 2013, 429 patients (20% of the overall study population) were included in the BALLET study in 52 hospitals in Spain, of whom 100 (23%) were ≥ 70 years. The median treatment duration was 3.14 and 3.03 months for exemestane and everolimus, respectively. The most common reasons for discontinuation of treatment were local reimbursement of everolimus (43%), followed by disease progression (31%) and the incidence of AEs (15%). The most frequent AEs causing permanent discontinuation were pneumonitis (4%), asthenia (2%) and stomatitis (2%). Overall, 87% of patients experienced at least one AE of any grade, 30% of patients at least one grade 3 AE and 2% of patients a grade 4 AE.
Conclusion
The safety profile in Spanish patients of the BALLET trial is consistent with the results obtained in the overall population of the trial, as well as in previous clinical trials.
Similar content being viewed by others
References
Gavila J, Lopez-Tarruella S, Saura C, Munoz M, Oliveira M, De la Cruz-Merino L, et al. SEOM clinical guidelines in metastatic breast cancer 2015. Clin Transl Oncol. 2015;17:946–55.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23:489–502.
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–9.
Nabholtz JM, Bonneterre J, Buzdar A, Robertson JF, Thurlimann B. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results. Eur J Cancer. 2003;39:1684–9.
Breast International Group 1–98 Collaborative G, Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57.
Buzdar AU, group At. ‘Arimidex’ (anastrozole) versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer—efficacy overview. J Steroid Biochem Mol Biol. 2003;86:399–403.
Giuliano M, Schifp R, Osborne CK, Trivedi MV. Biological mechanisms and clinical implications of endocrine resistance in breast cancer. Breast. 2011;20(Suppl 3):S42–9.
Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005.
Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–406.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39.
Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19.
Turner NC, Jiang Y, O’Leary B, Hrebien S, Cristofanilli M, Andre F, et al. Efficacy of palbociclib plus fulvestrant (P + F) in patients (pts) with metastatic breast cancer (MBC) and ESR1 mutations (mus) in circulating tumor DNA (ctDNA). J Clin Oncol. 2016;34:512. doi:10.1200/JCO.2016.34.15_suppl.512.
Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O’Reilly T, Evans DB, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–28.
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–84.
Beck JT, Hortobagyi GN, Campone M, Lebrun F, Deleu I, Rugo HS, et al. Everolimus plus exemestane as first-line therapy in HR(+), HER2(−) advanced breast cancer in BOLERO-2. Breast Cancer Res Treat. 2014;143:459–67.
Campone M, Bachelot T, Gnant M, Deleu I, Rugo HS, Pistilli B, et al. Effect of visceral metastases on the efficacy and safety of everolimus in postmenopausal women with advanced breast cancer: subgroup analysis from the BOLERO-2 study. Eur J Cancer. 2013;49:2621–32.
Gradishar WJ. Treatment challenges for community oncologists treating postmenopausal women with endocrine-resistant, hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Cancer Manag Res. 2016;8:85–94.
Jerusalem G, Mariani G, Ciruelos EM, Martin M, Tjan-Heijnen VC, Neven P, et al. Safety of everolimus plus exemestane in patients with hormone-receptor-positive, HER2-negative locally advanced or metastatic breast cancer progressing on prior non-steroidal aromatase inhibitors: primary results of a phase IIIb, open-label, single-arm, expanded-access multicenter trial (BALLET). Ann Oncol. 2016;27:1719–25.
Lueftner D, Schuetz F, Grischke E-M, Fasching PA, Wimberger P, Foerster FG, et al. Breast cancer treatment with everolimus and exemestane for ER + women: results of the first interim analysis of the noninterventional trial BRAWO. J Clin Oncol. 2014;32:578.
Rugo HS, Seneviratne L, Beck JT, Glaspy JA, Peguero JA, Pluard TJ, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18:654–62. doi:10.1016/S1470-2045(17)30109-2.
Cardoso F, Villanueva C, Royce M, Cruz F, Debled M, Hegg R, et al. Everolimus (EVE) plus endocrine therapy in patients with estrogen receptor–positive (ER +), human epidermal growth factor receptor 2-negative (HER2 −) advanced breast cancer (BC): First- and second-line data from the BOLERO-4 study. J Clin Oncol. 2017;35:1010.
Kornblum NS, Manola J, Klein P, Ramaswamy B, Brufsky A, Stella PJ et al., editors. PrECOG 0102: a randomized, double-blind, phase II trial of fulvestrant plus everolimus or placebo in post-menopausal women with hormone receptor (HR)-positive, HER2-negative metastatic breast cancer (MBC) resistant to aromatase inhibitor (AI) therapy. San Antonio Breast Cancer Symposium; 2016; San Antonio, Texas, EEUU.
Acknowledgements
The authors wish to thank Dr. Beatriz Gil-Alberdi from HealthCo Comunicación y Contenidos en Salud S. L. (Madrid, Spain) for her help in the preparation of the first draft of this manuscript. Novartis Spain provided financial support for medical writing services. The authors acknowledge the participation of the following oncologists in this study: Dr. Eva Ciruelos Gil, Hospital Universitario 12 de Octubre; Dr. Francisco Lobo Semper, Fundación Jiménez Díaz; Dra. Noelia Martínez Jañez, Hospital Universitario Ramón y Cajal; Dra. Pilar Zamora Auñón, Hospital Universitario La Paz; Dr. Álvaro Rodríguez-Lescure, Hospital Universitario de Elche; Dr. Francisco Ayala de la Peña, Hospital Morales Meseguer; Dr. José Ponce Lorenzo, Hospital Universitario de Alicante; Dr. Ignacio Chacón López-Muñiz, Hospital Virgen de la Salud; Dra. Aurelia Bustos Moreno, Hospital San Juan de Alicante; Dra. Mireia Margeli Vila, Hospital Germans Trials i Pujol; Dra. Sonia del Barco Berrón, Hospital Doctor Josep Trueta; Dr. Ignasi Tusquets Trías de Bes, Hospital del Mar; Dr. Joaquín Gavilá Gregori, Instituto Valenciano de Oncología; Dr. Diego Pérez Martín, Hospital Costa del Sol; Dra. Antonia Perelló Martorell, Hospital Son Espases; Dr. Jesús García Mata, Complejo Hospitalario de Ourense; Dr. Eduardo Martínez Dueñas, Hospital Provincial de Castellón; Dra. Silvia Antolín Novoa, Complejo Hospitalario A Coruña; Dr. Rafael López López, Complejo Hospitalario de Santiago; Dr. Antonio Antón Torres, Hospital Universitario Miguel Servet; Dra. Elena Galve Calvo, Hospital de Basurto; Dr. José Juan Illaramendi Mañas, Centro Hospitalario de Navarra; Dra. Isabel Álvarez, Hospital de Donostia; Dra. María García González, Hospital Universitario de Burgos; Dr. Francisco Carabantes Ocon, Hospital Carlos Haya; Dr. Rubén del Toro Salas, Hospital de Jerez; Dr. Juan Lucas Bayo Calero, Hospital Juan Ramón Jiménez; Dra. Pilar López Álvarez, Hospital Virgen de la Candelaria; Dra. María Lomas Garrido, Centro Hospitalario de Jaén; Dr. Norberto Batista López, Hospital Universitario de Canarias; Dr. Miguel Ángel Seguí, Hospital Parc Taulí; Blanca Cantos Sánchez, Hospital Puerta de Hierro; Dra. Montserrat Muñoz Mateu, Hospital Clinic de Barcelona; Dr. Agustí Barnadas i Molins, Hospital Sant Pau i Santa Creu; Dr. Miguel Martín Jiménez, Hospital Gregorio Marañón; Dr. Manuel Codes de Villena, Hospital Virgen de la Macarena; Dr. José Manuel Baena Cañada, Hospital Puerta del Mar; Dra. Vega Iranzo González Cruz, Hospital General de Valencia; Dr. José Ángel García Sáenz, Hospital Clínico San Carlos; Dra. Yolanda Fernández Pérez, Hospital Central de Asturias; Dra. Laura Murillo Jaso, Hospital Clínico Lozano Blesa; Dr. Santos Enrech, Hospital Universitario de Getafe; Dr. Juan de la Haba Rodríguez, Hospital Universitario Reina Sofía; Dr. Serafín Morales Murillo, Hospital Universitario Arnau de Vilanova; Dr. José Luis García Puche, Hospital Universitario San Cecilio; Dra. Isaura Fernández Pérez, Complejo Hospitalario de Vigo; Dra. Maria Vidal, Hospital Universitari Vall d’Hebron; Dr. Manuel Ramos Vázquez, Centro Oncológico de Galicia; Dra. Isabel Garau Llinàs, Hospital Son Llàtzer; Dra. Ana Miguel, Xarxa Assistencial de Manresa; Dra. Victoria Castellón Rubio, Hospital de Torrecárdenas; Dr. Miguel Ángel Lara, Hospital Infanta Leonor; Dra. Ana Lluch, Hospital Clínico de Valencia; Dra. Catalina Falo, Hospital Universitari de Bellvitge; Dra. Esperanza Blanco, Hospital Infanta Cristina; Dra. Susana Martínez Peralta, Hospital de Mataró.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest that may inappropriately influence this work.
Ethical statement
The study has been performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants of this study.
Rights and permissions
About this article
Cite this article
Ciruelos, E., Vidal, M., Martínez de Dueñas, E. et al. Safety of everolimus plus exemestane in patients with hormone-receptor-positive, HER2-negative locally advanced or metastatic breast cancer: results of phase IIIb BALLET trial in Spain. Clin Transl Oncol 20, 753–760 (2018). https://doi.org/10.1007/s12094-017-1784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1784-1