Abstract
Introduction
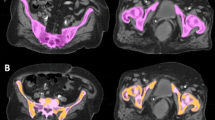
Hematologic toxicity (HT) in cervical cancer patients can cause treatment delays and reduction in chemotherapy, especially in high risk patients. Dose to PET-defined regions of active bone marrow (ABM) has been shown to correlate with cytopenias. An absolute volume of ABM spared may accurately represent hematopoietic reserve and risk of HT. This analysis evaluates whether the volume of ABM spared can more accurately predict HT compared to conventional dosimetric parameters.
Methods
Thirty-one patients treated for cervical cancer with chemoradiation from 9/2011 to 8/2016 were retrospectively reviewed. Receiver operating characteristic (ROC) curve were used to assess optimal cutpoint criterions for grade 3+ HT based on the CTCAEv4. Conventional dosimetric parameters to PBM and ABM (mean dose, V10, V20, V40) were assessed as well as the absolute volume (cc) of PBM and ABM spared 10, 20, and 40 Gy.
Results
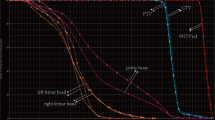
The absolute volume of PBM spared 10 Gy (< 230 cc; AUC 0.732, p = 0.03) as well as volume of ABM spared 10 Gy (< 179 cc; AUC 0.815, p = 0.0002), spared 20 Gy (< 186 cc; AUC 0.774, p = 0.0015), and spared 40 Gy (< 738 cc; AUC 0.887, p < 0.0001) all predicted grade 3+ HT. In patients with < 738 cc of ABM spared 40 Gy, 18/18 (100%) had grade 3+ toxicity compared to 6/13 (46%) of patients with > 738 cc of ABM spared 40 Gy (p < 0.0001).
Conclusion
The baseline volume of ABM and the fraction of ABM present in patients vary significantly. The ongoing NRG-GY006 trial and other efforts at bone marrow sparing use V10, V20, and mean dose to the ABM during planning optimization. This analysis suggests that the volume of ABM spared 40 Gy (> 738 cc) may be a stronger predictor of HT than conventional dosimetric parameters. This should be further evaluated for clinical use.



Similar content being viewed by others
References
Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013;63(2):88–105.
Klopp AH, et al. A phase III randomized trial comparing patient-reported toxicity and quality of life (QOL) during pelvic intensity modulated radiation therapy as compared to conventional radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96(2):S3.
Mell LK, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys. 2017;97(3):536–45.
Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Preliminary analysis of RTOG 9708: adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high-risk endometrial cancer. Int J Radiat Oncol Biol Phys. 2004;59(1):168–73.
Lee J, et al. Safety and efficacy of semiextended field intensity-modulated radiation therapy and concurrent cisplatin in locally advanced cervical cancer patients: an observational study of 10-year experience. Medicine (Baltimore). 2017;96(10):e6158.
Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2003;68(3):217–26.
Rose BS, et al. Correlation between radiation dose to (1)(8)F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(4):1185–91.
Liang Y, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys. 2013;85(2):406–14.
Protocol Development | CTEP. [Online]. Available:https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 15 June 2017.
Klopp AH, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86(1):83–90.
Zhu H, et al. Longitudinal study of acute haematologic toxicity in cervical cancer patients treated with chemoradiotherapy. J Med Imaging Radiat Oncol. 2015;59(3):386–93 (quiz 394).
Falcetta FS, Medeiros LR, Edelweiss MI, Pohlmann PR, Stein AT, Rosa DD. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. 2016;11:CD005342.
Mell LK, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol. 2006;66(5):1356–65.
Albuquerque K, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow? Sparing pelvic IMRT. Int J Radiat Oncol. 2011;79(4):1043–7.
Elicin O, et al. [18F]FDG-PET standard uptake value as a metabolic predictor of bone marrow response to? Radiation: impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int J Radiat Oncol. 2014;90(2)1099–107.
Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62(5):1371–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This study was an IRB approved retrospective study.
Rights and permissions
About this article
Cite this article
Zhou, Y.M., Freese, C., Meier, T. et al. The absolute volume of PET-defined, active bone marrow spared predicts for high grade hematologic toxicity in cervical cancer patients undergoing chemoradiation. Clin Transl Oncol 20, 713–718 (2018). https://doi.org/10.1007/s12094-017-1771-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1771-6