Abstract
Purpose
We compared the clinical efficacy and toxicity of stereotactic body radiotherapy with induction chemotherapy and concurrent radiochemotherapy vs stereotactic body radiotherapy with subsequent chemotherapy in patients with clinical stage T1-3N0M0 non-small cell lung carcinoma.
Methods
We retrospectively analyzed 38 patients with c-stage T1-3N0M0 non-small cell lung carcinoma who received stereotactic body radiotherapy. All patients received six cycles of chemotherapy. Fifteen of the patients were treated with three cycles of induction chemotherapy, one cycle of concurrent radiochemotherapy, and then two cycles of consolidation chemotherapy, while 23 patients received Sequential Radiotherapy/Chemotherapy.
Results
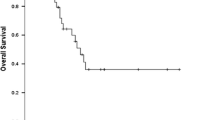
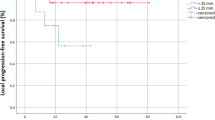
Patients in the induction chemotherapy group experienced a longer duration of esophagitis (median 2 vs 0, range 0–6 vs 0–3.6 weeks, p = 0.04). We divided the patients into two groups based on their median pre-treatment tumor volume (cm3): >32.11 and ≤32.11. The tumor response rate in patients with larger tumor volume was substantially higher in the induction chemotherapy group than in the Sequential Radiotherapy/Chemotherapy group (66.67 vs 40%). Among patients with pre-treatment tumor volume (cm3) >32.11, the median local progression-free survival (LPFS) in the induction chemotherapy group and Sequential Radiotherapy/Chemotherapy group was 18 months (range 7–72 months) and 11 months (range 6–53 months), respectively. There was a statistically significant difference between the two groups (p = 0.006).
Conclusions
Simultaneous SBRT and chemotherapy can result in a longer duration of esophagitis. However, for patients with large tumor volume, ICT combined with concurrent radiochemotherapy may result in better local tumor response as well as longer LPFS and progression-free survival. To better elucidate the best treatment, further clinical trials are needed.

Similar content being viewed by others
References
Ricardi U, Badellino S, Filippi AR. Stereotactic body radiotherapy for early stage lung cancer: history and updated role. Lung Cancer. 2015;90(3):388–96.
Kang KH, Okoye CC, Patel RB, et al. Complications from stereotactic body radiotherapy for lung cancer. Cancers (Basel). 2015;7(2):981–1004.
Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer [Adjuvant Navelbine International Trialist Association (ANITA)]: a randomised controlled trial. Lancet Oncol. 2006;7(9):719–27.
Tokuda Y, Takigawa N, Kozuki T, et al. Long-term follow-up of phase II trial of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small cell lung cancer. Acta Oncol. 2012;51(4):537–40.
Werner-Wasik M, Pequignot E, Leeper D, et al. Predictors of severe esophagitis include use of concurrent chemotherapy, but not the length of irradiatedesophagus: a multivariate analysis of patients with lung cancer treated with nonoperativetherapy. Int J Radiat Oncol Biol Phys. 2000;48(3):689–96.
Abelson JA, Murphy JD, Loo BW Jr, et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Dis Esophagus. 2012;25(7):623–9.
National Cancer Institute, Common terminology criteria for adverse events v4.0. NCI, NIH, DHHS. 2009. NIH publication 09–7473. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 30 Jan 2016
Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–101.
Chen Y, Guo W, Lu Y, et al. Dose-individualized stereotactic body radiotherapy for T1-3N0 non-small cell lung cancer: long-term results and efficacy of adjuvant chemotherapy. Radiother Oncol. 2008;88(3):351–8.
Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia groupB (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88(17):1210–5.
Semrau S, Bier A, Thierbach U, et al. Concurrent radiochemotherapy with vinorelbine plus cisplatin or carboplatin in patients with locally advanced non-small-cell lung cancer (NSCLC) and an increased risk of treatment complications. Preliminary results. Strahlenther Onkol. 2003;179(12):823–31.
Pöttgen C, Eberhardt WE, Gauler T, et al. Intensified high-dose chemoradiotherapy with induction chemotherapy in patients with locally advanced non-small-cell lung cancer-safety and toxicity results within a prospective trial. Int J Radiat Oncol Biol Phys. 2010;76(3):809–15.
Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J. Thorac. Oncol. 2012;7(3):542–51.
Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (pft) changes after stereotactic body radiotherapy (sbrt) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4(7):838–44.
Werner-Wasik M, Paulus R, Curran WJ Jr, et al. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non-small-cell lung cancer: analysis of the radiation therapy oncology group (RTOG) database. Clin Lung Cancer. 2011;12(4):245–51.
Jones GC, Kehrer JD, Kahn J, et al. Primary treatment options for high-risk/medically inoperable early stage NSCLC patients. Clin Lung Cancer. 2015;16(6):413–30.
Sause W, Kolesar P, Taylor S IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000;117(2):358–64.
Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–9.
Vokes EE, Herndon JE 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: cancer and Leukemia Group B. J Clin Oncol. 2007;25(13):1698–704.
Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer. 2016. doi:10.1002/cncr.30375.
Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys. 2011;80(5):1343–9.
Chi A, Liao Z, Nguyen NP, et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94(1):1–11.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has have been approved by the ethics committee of Shengjing Hospital of China Medical University. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Sun, Y., Duan, Q., Chen, X. et al. Comparative efficacy and toxicity of induction chemotherapy with concurrent stereotactic body radiotherapy and stereotactic body radiotherapy with subsequent chemotherapy in patients with clinical stage T1-3N0M0 non-small cell lung carcinoma. Clin Transl Oncol 19, 1498–1506 (2017). https://doi.org/10.1007/s12094-017-1694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1694-2