Abstract
Purpose
TAS-102 is a combination of the thymidine-based nucleoside analog trifluridine and the thymidine phosphorylase inhibitor tipiracil. Efficacy and safety of TAS-102 in patients with metastatic colorectal cancer (mCRC) refractory or intolerant to standard therapies were evaluated in the phase 3 RECOURSE trial. Results of RECOURSE demonstrated significant improvement in overall survival (OS) and progression-free survival (PFS) with TAS-102 versus placebo [hazard ratio (HR) = 0.68 and 0.48 for OS and PFS, respectively; both P < 0.001]. The current analysis evaluates efficacy and safety of TAS-102 in the RECOURSE Spanish subgroup.
Methods
Primary and key secondary endpoints were evaluated in a post hoc analysis of the RECOURSE Spanish subgroup, using univariate and multivariate analyses. Safety and tolerability were reported with descriptive statistics.
Results
The RECOURSE Spanish subgroup included 112 patients (mean age 61 years, 62 % male). Median OS was 6.8 months in the TAS-102 group (n = 80) versus 4.6 months in the placebo group (n = 32) [HR = 0.47; 95 % confidence interval (CI): 0.28–0.78; P = 0.0032). Median PFS was 2.0 months in the TAS-102 group and 1.7 months in the placebo group (HR = 0.47; 95 % CI: 0.30–0.74; P = 0.001). Eighty (100 %) TAS-102 versus 31 (96.9 %) placebo patients had adverse events (AEs). The most common drug-related ≥Grade 3 AE was neutropenia (40 % TAS-102 versus 0 % placebo). There was 1 (1.3 %) case of febrile neutropenia in the TAS-102 group versus none in the placebo group.
Conclusions
In the RECOURSE Spanish subgroup, TAS-102 was associated with significantly improved OS and PFS versus placebo, consistent with the overall RECOURSE population. No new safety signals were identified.
ClinicalTrials.gov study number
NCT01607957
Similar content being viewed by others
Introduction
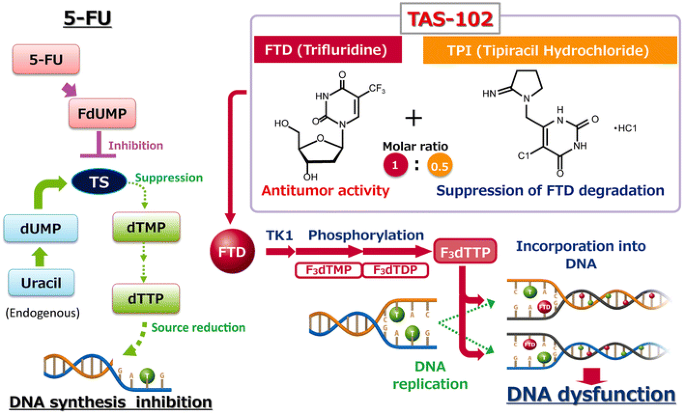
TAS-102 is an oral combination treatment consisting of trifluridine (FTD), a thymidine-based nucleoside analog, and tipiracil hydrochloride (TPI), at a molar ratio of 1:0.5 (weight ratio: 1:0.471). It is approved for use in Japan and the United States, and also has been recently approved in Europe. The primary cytotoxic mechanism of oral FTD is through incorporation into DNA following phosphorylation by thymidine kinase, leading to DNA dysfunction (Fig. 1) [1, 2]. This mechanism is distinct from that of 5-fluorouracil (5-FU) and other fluoropyrimidines that produce their cytotoxic effects through inhibition of thymidylate synthase. Although phosphorylated FTD does inhibit this enzyme, this activity is secondary to its direct effects on DNA when administered orally. This may explain the activity of TAS-102 in human cancer xenografts resistant to 5-FU [1, 3]. TPI improves the bioavailability of FTD by inhibiting its catabolism by thymidine phosphorylase [4]. The combination of these two agents makes TAS-102 an attractive candidate for treatment of patients with metastatic colorectal cancer (mCRC) who are refractory to fluoropyrimidines [5].
Comparison of the mechanisms of action of TAS-102 and 5-FU. 5-FU fluorouracil; dTMP and dTTP deoxythymidine mono- and triphosphate; dUMP deoxyuridine monophosphate; F 3 dTMP, F 3 dTDP, and F 3 dTTP trifluorodeoxythymidine mono-, di-, and triphosphate; FdUMP fluorodeoxyuridine monophosphate; TK1 thymidine kinase 1; TPase thymidine phosphorylase; TPI tipiracil hydrochloride; TS thymidylate synthase
TAS-102 has shown promise in a number of phase 1 and 2 trials [6–10]. A phase 2, double-blind, randomized, controlled trial conducted in Japanese patients with mCRC refractory to 5-FU, irinotecan, and oxaliplatin demonstrated a median overall survival (OS) of 9.0 months with TAS-102 compared with 6.6 months in the placebo group [hazard ratio (HR) = 0.56; 95 % confidence interval (CI), 0.39–0.81; P = 0.001]. More recently, in the phase 3 RECOURSE trial in patients with mCRC refractory to standard therapies, TAS-102 demonstrated a significant improvement compared with placebo in median OS (7.1 vs 5.3 months; HR = 0.68; 95 % CI, 0.58–0.81; P < 0.001) and progression-free survival (PFS) (2.0 vs 1.7 months; HR = 0.48; 95 % CI, 0.41–0.57; P < 0.001) [11].
The initial phase 2 trial of TAS-102 included only patients from Japan, and a subset of patients enrolled in RECOURSE were from Europe [9, 11]. It is of interest, therefore, to assess the efficacy of TAS-102 in patients enrolled in Spain, which accounted for a sizeable portion (14 %) of patients enrolled in the international RECOURSE trial. The objective of the current analysis was to evaluate the efficacy and safety of TAS-102 in the Spanish subgroup of patients enrolled in the RECOURSE trial.
Methods and patients
Study design
The protocol for the RECOURSE study has been described in detail previously [11]. Briefly, the RECOURSE trial was a global, multicenter, randomized, double-blind, placebo-controlled phase 3 trial comparing TAS-102 plus best supportive care with placebo plus best supportive care. Patients were randomly assigned in a 2:1 ratio to receive TAS-102 or placebo and were stratified according to KRAS status (wild type, mutant status), time since diagnosis of first metastasis (<18 months, ≥18 months), and geographic region [Japan or Western (United States, Europe, and Australia combined)]. This analysis focuses on those patients in the European stratum who were randomized in Spain. Subjects were randomized at 11 different centers in Spain. This trial is registered with ClinicalTrials.gov number, NCT01607957.
The study was conducted according to the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines, and the study was approved by institutional review boards at each participating center. All patients provided written informed consent.
Patients
Eligible patients with metastatic, biopsy-documented adenocarcinoma of the colon or rectum who had received ≥2 prior lines of therapy with standard chemotherapies and had been refractory to antitumor therapy were eligible for randomization. Prior therapy could have included adjuvant treatment if tumor recurrence had occurred within 6 months, tumor progression within 3 months after the last administration of therapy, or if clinical adverse events precluded rechallenge with standard chemotherapy. Knowledge of KRAS status was required, and patients must have received prior chemotherapy with a fluoropyrimidine, oxaliplatin, irinotecan, and bevacizumab, and cetuximab or panitumumab if they had KRAS wild-type tumors. In addition, patients had to be ≥18 years of age, have adequate bone-marrow, liver, and renal function, and have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were centrally randomized via an Interactive Voice/Web Response System based on a dynamic allocation method.
Endpoints
The primary endpoint was OS, which was defined as the time from randomization to death from any cause. Secondary endpoints included PFS, which was defined as the time from randomization to the first radiographic confirmation of disease progression or death from any cause, overall response rate (the proportion of patients with a complete or partial response), disease control rate (the proportion of patients with a complete or partial response, or stable disease, with stable disease assessed at least 6 weeks after starting treatment), time to deterioration of ECOG performance status to 2 or greater, and safety. Radiographic assessments were conducted every 8 weeks, and treatment was continued until disease progression as defined by RECIST (version 1.1) [12], clinical progression, development of serious adverse events, study withdrawal, death, or a decision by the physician that discontinuation was in the patient’s best interest. Adverse events were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
The study protocol included a prespecified analysis of outcomes and safety according to geographic subregion. The same methodology was used for this post hoc analysis of the Spanish subgroup. OS and PFS were analyzed in the intent-to-treat population with the use of a two-sided, stratified log-rank test, with the HR and two-sided 95 % confidence intervals based on a stratified Cox proportional hazards model and the associated Kaplan–Meier survival estimates. The median follow-up time for survival was calculated by means of the reverse Kaplan–Meier method. Objective response and disease control rates were compared using the Fisher’s exact test in the subgroup of patients in the intent-to-treat population who had measurable disease at baseline. The time to deterioration of ECOG performance status to 2 (defined as: ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50 % of waking hours) was analyzed with a similar approach to that employed for analysis of OS. Adverse events and laboratory abnormalities were summarized for all patients who received at least one dose of study drug. The number of hospitalizations, number and percentage of patients hospitalized, reason for hospitalization, and the total duration of hospitalization were summarized descriptively by treatment group.
Results
Patients
Of the 112 patients randomized to the RECOURSE study in Spain, 80 were in the TAS-102 arm and 32 in the placebo arm. The Spanish subgroup, therefore, represents 14 % of the overall RECOURSE population and 28 % of the prespecified European stratum. Patient and tumor characteristics for subjects enrolled and randomized in Spain are shown in Table 1, along with characteristics for the overall population. Because patients enrolled in Spain represented a significant portion of the overall and prespecified European stratum, baseline characteristics were well balanced between the two arms. However, there were some differences between the Spanish and overall populations: the Spanish subgroup was entirely Caucasian vs 57.6 % of the overall population; Spanish patients had worse ECOG performance status with 68.8 % having a performance status of 1 vs 44.0 % in the overall population (31.3 and 56.0 % had performance status of 0 in the two populations, respectively); and more patients in the Spanish subgroup had had prior regorafenib treatment (25.9 vs 18.0 %).
Efficacy
The median OS in the Spanish subgroup was 6.8 months in the TAS-102 group, significantly greater than the 4.6 months observed in the placebo group (HR = 0.47; 95 % CI, 0.28–0.78; P = 0.0032) (Fig. 2a; Table 2). The 1-year OS rate was 27.5 % (95 % CI, 14.0–42.9) in the TAS-102 arm and 20.4 % (95 % CI, 7.9–36.9) in the placebo arm (Fig. 2b). Similarly, median PFS in the TAS-102 group from in Spanish patients was 2.0 months, significantly longer than 1.7 months observed in the placebo group (HR = 0.47; 95 % CI, 0.30–0.74; P < 0.001) (Fig. 3; Table 2). As shown in Figs. 2, 3, and Table 2, these results were consistent with the OS and PFS reported for the overall population.
There was no significant difference between arms in terms of the best overall response rate and the disease control rate in the Spanish subgroup. No patients in either group had a complete response. The TAS-102 arm had a partial response rate of 3.9 % (3 patients) vs 0 % in the placebo arm. The disease control rate was 39.5 and 19.4 % in the TAS-102 and placebo arms, respectively. The median time to deterioration of ECOG performance status to 2 was 5.4 months for the TAS-102 group versus 3.3 months for the placebo group (HR = 0.31; 95 % CI, 0.18–0.54; P < 0.0001). These results were consistent with response and disease control rates in the overall population.
Safety and tolerability
Overall Grade ≥3 adverse events in the Spanish subgroup, regardless of causation, occurred in 72.5 % of patients in the TAS-102 arm and 56.3 % in the placebo arm. The most common nonhematologic adverse events of any grade seen with TAS-102 in ≥30 % of the Spanish subgroup were asthenia, nausea, decreased appetite, and diarrhea (Table 3). Asthenia and back pain were the most common (≥5 %) nonhematologic Grade ≥3 adverse events seen with TAS-102. The incidence of asthenia and mucosal inflammation was somewhat higher among Spanish patients than in the overall population and the incidence of fatigue was somewhat lower, but there were no other notable differences in the incidence of individual adverse events.
There were similar incidences of laboratory abnormalities in the Spanish population compared with the overall population. For Grade ≥3 laboratory abnormalities, myelosuppression was common with TAS-102, with 40 and 16.3 % experiencing neutropenia or leukopenia, compared with no patients in the placebo arm; in addition, 13.8 % experienced anemia, compared with 6.3 % in the placebo arm. Lymphocytopenia and thrombocytopenia were also higher in the TAS-102 arm compared with the placebo arm. Total bilirubin and alkaline phosphatase were elevated in a substantial portion of the Spanish population in both treatment groups (Table 3).
Importantly, the rate of serious adverse events and hospitalizations was lower in the TAS-102 arm than in the placebo arm: 20 and 37.5 % of patients experienced serious adverse events in the TAS-102 and placebo arms, respectively. A similar proportion of patients were hospitalized during the study, with the vast majority being due to serious adverse events; one patient in the TAS-102 arm was hospitalized due to febrile neutropenia and one patient in the placebo arm was hospitalized due to health deterioration (Table 3). Overall, TAS-102 was considered to be well tolerated in the Spanish population.
Discussion
The results observed in the Spanish subgroup (14 % of the total study population) were consistent with the results of the overall RECOURSE study. Indeed, TAS-102 was associated with significant improvements in OS and PFS in the Spanish population. In Spanish patients, the risk of death and risk of disease progression or death were both reduced by 53 % with TAS-102 compared with placebo (P = 0.0032 and P = 0.001, respectively). The reduction in the risk of death with TAS-102 in this population was greater than that observed in the overall population, with an HR of 0.47 (95 % CI: 0.28–0.78) in the Spanish group and 0.68 (0.58–0.81) in the overall population [11]. The HR of OS in the Spanish subgroup was lower than that of the overall RECOURSE population. This may be explained by the fact that the Spanish population had a substantially higher percentage of patients with an ECOG status of 1 and, therefore, a worse prognosis. The Kaplan–Meier curves are largely coincident for the TAS-102 arms in Spanish patients and overall (Fig. 2). There was no significant benefit in favor of TAS-102 in terms of best overall response and disease control rates, although both showed trends favoring TAS-102. Similar results were seen for the Phase 3 trial of regorafenib which noted similar OS and PFS for Japanase and primarily Caucasian non-Japanese patients [13]. A recent analysis of European cancer registries showed that the mean 5-year OS for colorectal and rectal cancers in Spanish patients was similar to the European mean for those cancers, with mean OS in Southern Europe being similar to that of Northern and Central Europe, and greater than that in the UK, Ireland, and Eastern Europe [14]. This suggests that survival for mCRC in Spanish patients is representative of Europe as a whole and that the results presented here corroborate results for the whole continent [15].
There were a number of differences in the baseline characteristics of the Spanish population when compared with the overall RECOURSE population, as might be expected in a multicenter international study that included patients from Europe, Asia, the United States, and Australia. All of the patients from Spain were Caucasian, compared with 57.6 % of the overall population (34.8 % of the patients in the trial were Asian). The demonstrated efficacy of TAS-102 in Caucasian Spanish patients is encouraging for the generalizability of the results of the RECOURSE study to different populations. This is significant because the phase 2 trial of TAS-102 in mCRC was conducted solely in Japanese patients [9], and differences in response have been noted previously between Western and Japanese subjects for gefitinib in lung cancer [16]. In the prespecified analysis of regional subgroups in RECOURSE, the OS difference between TAS-102 and placebo groups in the Japanese population (HR: 0.75, 95 % CI: 0.57–1.00) was not as pronounced as in the US (HR: 0.56, 95 % CI: 0.34–0.94) or European (HR: 0.62, 95 % CI: 0.48–0.80) populations, which were largely composed of Caucasian patients. Demographics as well as the small size of the Spanish subgroup relative to the overall study may explain the apparent survival benefit in the Spanish population.
There was some imbalance of colon cancer versus rectal cancer in the placebo group enrolled in Spain, compared with the TAS-102 arm and the overall population. Although patients in the Spanish subgroup had similar time from diagnosis, number of rounds of prior chemotherapy, and KRAS mutation prevalence as the overall RECOURSE population, the Spanish subpopulation had 50 % higher incidence of ECOG performance status of 1 than the overall study population, which indicates poorer health status. This higher incidence of a worse ECOG performance status would be expected to have a negative impact on outcomes since performance status on its own has been shown to be a good prognostic factor for patients with advanced cancer [17]. Despite this, the time to deterioration to performance status ≥2 in Spanish patients was similar to that observed in the overall population for both TAS-102 (5.4 months versus 5.7 months) and placebo (3.3 vs 4.0 months) [11]. The reasons for this discrepancy in the ECOG performance status of Spanish patients and the overall population are unclear. However, they are consistent with a similar analysis of the Phase 3 CORRECT trial of regorafenib which noted a higher rate of ECOG performance status of 0 among Japanese patients compared to the primarily Caucasian non-Japanese population [13]. It is possible, therefore, that regional or cultural differences in interpretation of ECOG performance status definitions had a role.
TAS-102 was generally well tolerated in this subgroup of patients from the RECOURSE study, with the overall safety profile being similar to that of the overall population. Importantly, no new safety signals were seen in this Spanish population. As with the overall study, neutropenia was the most frequently observed, clinically meaningful laboratory abnormality or adverse event, with one patient being hospitalized for febrile neutropenia. The only substantial difference reported in the safety profile of TAS-102 between the Spanish and overall RECOURSE populations was a higher incidence of asthenia and lower incidence of fatigue in Spanish patients. This difference is likely due to subtle local differences in interpretation of the definition of these two overlapping adverse events [18]. The rate of serious adverse events and hospitalizations in the Spanish subgroup was somewhat lower than in the overall population, but this could merely reflect local differences in healthcare expenditure, socioeconomic status, lifestyle, and general health status of the population [14], as well as the relatively low number of patients in this cohort. The lack of difference in adverse events differs from that seen in the recent trial of regorafenib, which noted a higher incidence of certain adverse events among Japanese patients than non-Japanese patients—including a higher incidence of Grade 3 adverse events [13].
The data presented here are limited by the fact that this was an unplanned post hoc analysis of the subgroup of patients who were randomized in Spain. As such, this analysis may not have had sufficient statistical power to draw definitive conclusions about the efficacy of TAS-102. However, this population represents a substantial proportion of the European geographic region, an analysis of which was preplanned. Furthermore, the Spanish population was larger than the American and Australian geographic subregions for which there were preplanned analyses.
The results of this analysis of a subgroup of Spanish patients with mCRC refractory to standard therapies who were enrolled in the international, multicenter, randomized, placebo-controlled RECOURSE study indicate that the results of the larger trial are generally applicable in this subpopulation. TAS-102 was found to impart significant improvements in OS and PFS compared with placebo, similar to that seen in the overall trial. TAS-102 was generally well tolerated in this population of Spanish patients, with no new safety signals unique to this particular population identified. TAS-102 may be a good treatment option for Spanish patients with mCRC who are refractory to standard treatments.
References
Emura T, Murakami Y, Nakagawa F, Fukushima M, Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Med. 2004;13(4):545–9.
Tanaka N, Sakamoto K, Okabe H, Fujioka A, Yamamura K, Nakagawa F, et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep. 2014;32(6):2319–26. doi:10.3892/or.2014.3487.
Nukatsuka M, Nakagawa F, Saito H, Sakata M, Uchida J, Takechi T. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, with irinotecan hydrochloride on human colorectal and gastric cancer xenografts. Anticancer Res. 2015;35(3):1437–45.
Emura T, Suzuki N, Fujioka A, Ohshimo H, Fukushima M. Potentiation of the antitumor activity of alpha, alpha, alpha-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol. 2005;27(2):449–55.
Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–87. doi:10.1056/NEJMra040958.
Overman MJ, Kopetz S, Varadhachary G, Fukushima M, Kuwata K, Mita A, et al. Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest. 2008;26(8):794–9. doi:10.1080/07357900802087242.
Overman MJ, Varadhachary G, Kopetz S, Thomas MB, Fukushima M, Kuwata K, et al. Phase 1 study of TAS-102 administered once daily on a 5-day-per-week schedule in patients with solid tumors. Invest New Drugs. 2008;26(5):445–54. doi:10.1007/s10637-008-9142-3.
Doi T, Ohtsu A, Yoshino T, Boku N, Onozawa Y, Fukutomi A, et al. Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer. 2012;107(3):429–34. doi:10.1038/bjc.2012.274.
Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi:10.1016/S1470-2045(12)70345-5.
Bendell JC, Rosen LS, Mayer RJ, Goldman JW, Infante JR, Benedetti F, et al. Phase 1 study of oral TAS-102 in patients with refractory metastatic colorectal cancer. Cancer Chemother Pharmacol. 2015;76(5):925–32. doi:10.1007/s00280-015-2850-4.
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–19. doi:10.1056/NEJMoa1414325.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi:10.1016/j.ejca.2008.10.026.
Yoshino T, Komatsu Y, Yamada Y, Yamazaki K, Tsuji A, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs. 2015;33(3):740–50. doi:10.1007/s10637-014-0154-x Epub 2014 Sep 12.
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, EUROCARE Working Group, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 2014;15(1):23–34. doi:10.1016/S1470-2045(13)70546-1.
Ohtsu A, Yoshino T, Wabha MM, Benedetti FM, Mayer RJ, van Cutsem E, et al; RECOURSE Study Group. Phase 3 RECOURSE trial of TAS-102 versus placebo with best supportive care in patients with metastatic colorectal cancer: geographic subgroups. 2015 American Society of Clinical Oncology Annual Meeting; 29 May-2 June, 2015; Chicago, IL. Abstract no. 3564. http://meetinglibrary.asco.org/content/148025-156. Accessed 4 Feb 2016.
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237–46. doi:10.1200/JCO.2003.10.038.
Jang RW, Caraiscos VB, Swami N, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10(5):e335–41.
Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71(7):1327–36.
Acknowledgments
The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this publication. All authors contributed to the research, writing, and reviewing of all drafts of this manuscript. All authors approved the final draft. Editorial support in the preparation of this publication was provided by Phase Five Communications, supported by Taiho Oncology Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author A reports employment at Taiho Oncology Inc. Author B reports providing scientific advice to Bayer. Author C reports consulting/advisory fees from Amgen, Boehringer Ingelheim, Celgene, Chugai, Imclone Systems Inc., Eli Lilly and Company, Merck & Co., Merck Serono, Millennium, Novartis, F. Hoffmann-La Roche Ltd., Sanofi, Symphogen, and Taiho Oncology Inc. All other authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
On behalf of the RECOURSE Study Group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Longo-Muñoz, F., Argiles, G., Tabernero, J. et al. Efficacy of trifluridine and tipiracil (TAS-102) versus placebo, with supportive care, in a randomized, controlled trial of patients with metastatic colorectal cancer from Spain: results of a subgroup analysis of the phase 3 RECOURSE trial. Clin Transl Oncol 19, 227–235 (2017). https://doi.org/10.1007/s12094-016-1528-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1528-7