Abstract
Purpose
Dose–volume histogram (DVH) has become an important tool for evaluation of radiation outcome as reflected from many clinical protocols. While dosimetric accuracy in treatment planning system (TPS) is well quantified, the variability in volume estimation is uncertain due to reconstruction algorithm that is investigated in this study. In addition, the impact of dose distribution and tumor control probability (TCP) were also investigated with CT slice thickness for IMRT planning.
Materials and methods
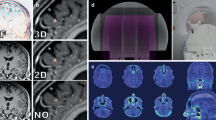
A water phantom containing various objects with accurately known volume ranging from 1 to 100 cm3 was scanned with 1, 2, 3, 5, and 10 mm slice thickness. The CT data sets were sent to Eclipse TPS for contour delineation and volume estimation. The data were compared with known volume for the estimation of error in the volume of each structure. IMRT Plans were generated on phantom containing four objects with different slice thickness (1–5 mm) to calculate TCP. ICRU-83-recommended dose points such as D 2%, D 50%, D 98%, as well as homogeneity and conformity index were also calculated.
Results
The variability of volumes with CT slice thickness was significant especially for small volume structures. A maximum error of 92 % was noticed for 1 cm3 volume of object with 10 mm slice thickness, whereas it was ~19 % for 1 mm slice thickness. For 2 and 3 cm3 objects, the maximum error of 99 % was noticed with 10 mm slice thickness and ~60 % with 5 mm. The differences are smaller for larger volumes with a cutoff at about 20 cm3. The calculated volume of the objects is a function of reconstruction algorithm and slice thickness. The PTV mean dose and TCP decreased with increasing slice thickness. Maximum variation of ~5 % was noticed in mean dose and ~2 % in TCP with change in slice thickness from 1 to 5 mm. The relative decrease in target volume receiving 95 % of the prescribed dose is ~5 % with change in slice thickness from 1 to 5 mm. The homogeneity index increases up to 163 % and conformity index decreases by 4 % between 1 and 5 mm slice thickness, producing highly inhomogeneous and least conformal treatment plan.
Conclusions
Estimation of a volume is dependent on CT slice thickness and the contouring algorithm in a TPS. During commissioning of TPS and for all clinical protocols, evaluation of volume should be included to provide the limit of accuracy in DVH from TPS, especially for small objects. A smaller slice thickness provides superior dosimetry with improved TCP. Thus, the smallest possible slice thickness should be used for IMRT planning, especially when smaller structures are present.







Similar content being viewed by others
References
Van Dyk J, Barnett RB, Cygler JE, Shragge PC. Commissioning and quality assurance of treatment planning computers. Int J Radiat Oncol Biol Phys. 1993;26:261–73.
IAEA TRS 430. Commissioning and quality assurance of computerized planning systems for radiation treatment of cancer Vienna, Austria: International Atomic Energy agency; 2004.
Eisbruch A, Foote RL, O’Sullivan B, Beitler JJ, Vikram B. Intensity-modulated radiation therapy for head and neck cancer: emphasis on the selection and delineation of the targets. Semin Radiat Oncol. 2002;12:238–49.
ICRU Report 50. Prescribing, recording, and reporting photon beam therapy. Report. Bethesda, MD: International Commission on Radiation Units and Measurements. 1993. ICRU Report 50.
ICRU 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50). report. Bethesda, MD: International Commission on Radiation Units and Measurements; 1999.
Muren LP, Karlsdottir A, Kvinnsland Y, Wentzel-Larsen T, Dahl O. Testing the new ICRU 62 ‘planning organ at risk volume’ concept for the rectum. Radiother Oncol. 2005;75:293–302.
Ahnesjö A, Aspradakis MM. Dose calculations for external photon beams in radiotherapy. Phys Med Biol. 1999;44:R99–155.
Ahnesjö A, Weber L, Murman A, Saxner M, Thorslund I, Traneus E. Beam modeling and verification of a photon beam multisource model. Med Phys. 2005;32:1722–37.
Fogliata A, Nicolini G, Vanetti E, Clivio A, Cozzi L. 2006; Dosimetric validation of the anisotropic analytical algorithm for photon dose calculation: fundamental characterization in water. Phys Med Biol. 2006;51:1421–38.
Knöös T, Ceberg C, Weber L, Nilsson P. The dosimetric verification of a pencil beam based treatment planning system. Phys Med Biol. 1994;39:1609–28.
Nisbet A, Beanage I, Vollmar H-S, Irvine C, Morgan A, Thwaites DI. Dosimetric verification of a commercial collapsed cone algorith in simulated clinical situations. Radiother Oncol. 2004;73:79–88.
Van Esch A, Tillikainen L, Pyykkonen J, Tenhunen M, Helminen H, Siljamaki S, et al. Testing of the analytical anisotropic algorithm for photon dose calculation. Med Phys. 2006;33:4130–48.
Ibbott GS, Haworth A, Followill DS. Quality assurance for clinical trials. Front Oncol. 2013;2013:3.
Ibbott GS, Followill DS, Molineu HA, Lowenstein JR, Alvarez PE, Roll JE. Challenges in credentialing institutions and participants in advanced technology multi-institutional clinical trials. Int J Radiat Oncol Biol Phys. 2008;71:S71–5.
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37:4078–101.
TG-53. American association of physicists in medicine radiation therapy committee task group 53: quality assurance for clinical radiotherapy treatment planning. Med Phys. 1998;1998(25):1773–829.
Ebert MA, Haworth A, Kearvell R, Hooton B, Hug B, Spry NA, et al. Comparison of DVH data from multiple radiotherapy treatment planning systems. Phys Med Biol. 2010;55:N337–46.
Henriquez FC, Vargas Castrillon S. Confidence intervals in dose volume histogram computation. Med Phys. 2010;2010(37):1545–53.
Lee CT, Dong L, Ahamad AW, Choi H, Cheung R, Lee AK, et al. Comparison of treatment volumes and techniques in prostate cancer radiation therapy. Am J Clin Oncol. 2005;28:618–25.
Fiorentino A, Caivano R, Pedicini P, Fusco V. Clinical target volume definition for glioblastoma radiotherapy planning: magnetic resonance imaging and computed tomography. Clin Transl Oncol. 2013;15:754–8.
Cheng CW, Das IJ. Treatment plan evaluation using dose-volume histogram (DVH) and spatial dose-volume histogram (zDVH). Int J Radiat Oncol Biol Phys. 1999;43:1143–50.
Prabhakar R, Ganesh T, Rath GK, Julka PK, Sridhar PS, Joshi RC, et al. Impact of different CT slice thickness on clinical target volume for 3D conformal radiation therapy. Med Dosim. 2009;34:36–41.
Prionas ND, Ray S, Boone JM. Volume assessment accuracy in computed tomography: a phantom study. J Appl Clin Med Phys. 2010;11(2):3037.
ICRU Report 83. Prescribing, recording, and reporting intensity-modulated photon- beam therapy (IMRT) (ICRU Report 83) report. Bethesda, MD: International Commission on Radiation Units and Measurements; 2010.
Schultheiss TE, Orton CG, Peck RA. Models in radiotherapy: volume effects. Med Phys. 1983;10:410–5.
Das IJ, Bieda M, Cheng C, Chopra K, Hasson B, Olch A, et al. Dosimetric comparison of inverse treatment planning system for IMRT: a collaborative study. Med Phys. 2004;31:1750.
Somigliana A, Zonca G, Loi G, Sichirollo AE. How thick should CT/MR slices to plan conformal radiotherapy? A study on the accuracy of three-dimensional volume reconstruction. Tumori. 1996;82:470–2.
Caivano R, Fiorentino A, Pedicini P, Califano G, Fusco V. The impact of computed tomography slice thickness on the assessment of stereotactic, 3D conformal and intensity-modulated radiotherapy of brain tumors. Clin Transl Oncol. 2013;2013:1–6.
Chao KSC, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. 2004; Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59:43–50.
Lok BH, Setton J, Caria N, Romanyshyn J, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiation therapy in oropharyngeal carcinoma: effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Srivastava, S.P., Cheng, CW. & Das, I.J. The effect of slice thickness on target and organs at risk volumes, dosimetric coverage and radiobiological impact in IMRT planning. Clin Transl Oncol 18, 469–479 (2016). https://doi.org/10.1007/s12094-015-1390-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1390-z