Abstract
Background
Bladder cancer is the second most common urological malignancy around the world and is by far the most frequent urological malignancy in China. Embelin is an active compound identified as a novel X-chromosome-linked IAP (XIAP) inhibitor from the Embelia ribes that exhibits various medicinal effects including anti-inflammatory and anti-cancer activities. However, therapeutic effect of Embelin to human bladder cancer is not yet determined.
Methods
We evaluated the sensitizing potential of Embelin on inhibiting cell growth and migration of bladder cancer cell line by CCK8, Transwell, and Western Blot, and explored its related mechanism. We performed IHC staining of XIAP in 35 bladder cancer tissues and corresponding adjacent non-neoplastic tissues.
Results
XIAP was significantly upregulated in bladder cancer cases. When the concentration of Embelin was used respectively at 5, 10, 20, 25, and 35 µmol/l, the survival of both T24 and 5637 cells decreased in a dose-/time-dependent manner. Our study confirmed that with the increase of concentration of Embelin, the expression levels of PI3K and p-Akt decreased significantly which further confirmed that Embelin inhibits cell growth by inducing apoptosis via PI3K/Akt pathway.
Conclusions
Embelin may be developed into a novel and potential chemotherapeutic drug for bladder cancer.
Similar content being viewed by others
Introduction
Bladder cancer is by far the most frequent urological malignancy in China [1]. Approximately 70 % of patients are diagnosed as non-muscle invasive bladder cancer (NMIBC) and could be treated by transurethral resection; however, most of NMIBC patients still recur; moreover, approximately 20 % of the bladder cancer patients present with muscle invasive bladder cancer (MIBC) associated with a strong propensity toward deadly metastases [2]. Despite years of intensive efforts on surgical techniques and adjuvant chemotherapy, bladder cancer remains a highly prevalent and lethal malignancy, which is highly refractory to the drug therapy [3, 4]. Therefore, new treatments are essential for increasing the life expectancy of bladder cancer patients.
Inhibitor of apoptosis proteins (IAPs), which are frequently over expressed in various tumors, have become promising targets for developing anti-cancer drugs [5, 6]. X-chromosome-linked IAP (XIAP) is one of the most potent inhibitors. XIAP, which can regulate cell apoptosis via inhibiting activity of caspase pathways, is most extensively studied and well characterized among the mammalian IAPs. Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone), one of small-molecule inhibitors of XIAP, is reported to possess anti-oxidant, anti-bacterial, and anti-inflammatory activities [7–9]. Embelin was identified as a small molecular weight inhibitor of XIAP which has been identified as one of the most potent inhibitors of caspase activity and apoptosis. The anti-apoptotic activity of XIAP is at least partially due to its ability to inhibit both mitochondrial-dependent and mitochondrial-independent apoptotic pathways by binding to and inhibiting the activation of initiator caspase-9, as well as the effector caspases (caspase-3 and -7), which are vital for the execution of apoptosis. Embelin is identified as a novel XIAP inhibitor from the Embelia ribes that exhibits various medicinal effects including anti-inflammatory and anti-cancer activities. However, the effect of Embelin on bladder cancer was not investigated yet. Therefore, we examined if Embelin could be a therapeutic agent for bladder cancer.
Materials and methods
Patients and tissue samples
The human clinical samples were collected from surgical specimens from 35 patients with bladder cancer who had received cystectomy or transurethral resection of bladder tumor (TUR-BT) at Nanfang Hospital, Southern Medical University between June 2010 and July 2014. The corresponding adjacent non-neoplastic tissues from the macroscopic tumor margin were isolated at the same time and used as controls. The specimens were staged according to the American Joint Committee on Cancer-Union Internationale Contre le Cancer tumor-node-metastasis (TNM) classification and histologically graded. Our study was approved by the Bioethics Committee of Southern Medical University; written prior informed consent and approval were obtained from all patients.
Reagents
Human urinary bladder transitional cell carcinoma (T24, 5637) cells were obtained from Chinese Academy of Science (Shanghai, China). Embelin was purchased from Abcam (Cambridge, UK). Rabbit antibodies of PI3K, Akt, p-Akt, and XIAP were purchased from Abcam (Cambridge, UK).
Cell culture and research methods
The T24 and 5637 cell lines were maintained in RPMI 1640 medium supplemented with 10 % fetal bovine serum (FBS). All media contained 100 units of penicillin/mL and 100 μg of streptomycin/ml. All cell lines were maintained in a humidified incubator at 5 % CO2 and 37 °C. The cells that entered the logarithmic growth period were selected for experiment. We selected different concentrations of the Embelin group, meanwhile setting DMSO blank control group. The experiment time was as followed: 12, 24, and 48 h. All trials were repeated three times.
Immunohistochemical staining
All specimens were fixed in 10 % buffered formalin for 24 h and embedded in paraffin. Sections (4-μm) were placed on silane-coated slides. After deparaffinization and rehydration, slides were placed into 3 % hydrogen peroxidase for 15 min, and were autoclaved at 121 °C in citrate buffer (10 mM, pH 6.0) for 10 min for antigen activation. After cooling at room temperature (RT) for 20 min, specimens were incubated with each blocking buffer for 15 min at RT, and were then incubated with an anti-XIAP antibody (BD Biosciences) at 4 °C overnight. Immunohistochemical staining was performed using a standard avidin–biotin complex method with a streptavidin–biotin–peroxidase kit (Nichirei, Tokyo, Japan). 3,3′-diaminobenzidine was used as a chromogen. Counterstaining was performed with hematoxylin. Negative controls were treated without antibody. Two independent pathologists blinded to the clinicopathologic variables evaluated the immunostaining. The immunostaining score was evaluated based on the whole slide. The results were recorded by assessing the percentage of positive cells and the intensity of the staining (1, mild; 2, moderate; and 3, intense) on the tumoral and the non-tumoral areas for each slide.
Measurement of the survival rates of cells with CCK-8 assay
A total of 5 × 103 cells were seeded into 96-well plates and cultured overnight with 200 μl each well, and added to the culture medium containing agents of different concentrations or control PBS with 100 μl each well, each concentration for parallel 4 wells after adherence. After culturing for 12, 24, and 48 h, the medium was replaced with 100 μl fresh medium and 10 μl CCK-8 solution. The cells were incubated for additional 4 h, and the absorbance was measured at 450 nm by an Enspire microplate reader (PerkinElmer, USA). The cell viability and IC50 values were calculated. Survival rate of tumor cells (%) = experimental group A value/control group A value × 100 %.
Determination of cell apoptosis by flow cytometry
After culturing for 12, 24, and 48 h, apoptosis was detected using the Annexin V-FITC Apoptosis Detection Kit. Cells were detached by trypsinization and washed three times in PBS, centrifuged at 1000×g for 5 min, and resuspended in 195 μl Annexin V-FITC binding buffer. 5 μl Annexin V-FITC was added and mixed. Then, the cells were stained in the dark for 10 min at room temperature. After that, cells were centrifuged at 1000×g for 5 min and resuspended in 190 μl of Annexin V-FITC binding buffer. Last, 10 μl propidium iodide staining solution was added and mixed. The cells were kept on ice in the dark and immediately subjected to flow cytometry analysis. The data were analyzed using the Cell Quest software. The experiment was repeated three times.
Cell migration assay
Transwell insert with a pore size of 8 lm from Corning, Inc. (Corning, NY) was used to determine tumor cell migration capacity. Cells were first transfected with miR-335 mimic or control RNA. After 24 h, the cells were starved in medium without fetal calf serum (FCS) for 24 h, and then the cells were resuspended in the FCS-free medium and placed in the top chambers in triplicate. The cells remaining on the upper membrane were removed with cotton wool, whereas the cells that had migrated to the bottom of the membrane were then fixed with 95 % ethanol and stained with 0.1 % crystal violet. Five visual fields of each insert were randomly chosen and photographed under a light microscope at 200× magnification. All experiments were performed in triplicate.
Protein extraction and Western blot
Bladder cancer cells were seeded onto six-well plates the day before transfections were performed. Forty-eight hours after transfection, cells were lysed with RIPA buffer containing protease inhibitors (Beyotime biotechnology, Haimen, China). The concentrations of the proteins were determined using a BCA Protein Assay Kit (Beyotime biotechnology, Haimen, China). Samples were thawed in 5 × SDS–PAGE sample loading buffer, vortexed, and then denatured at 100 °C for 5 min and placed on ice for 5 min. Cell lysates (approximately 30 μg of protein) were loaded on an 8 % SDS–PAGE gel and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) by Wet Electrophoretic Transfer (Bio-Rad Laboratories). The membranes were blocked for 1 h at room temperature, and incubated overnight at 4 °C with primary antibody in Tris-buffered saline with 0.05 % tween (TBST) containing 5 % non-fat milk. After washing three times with TBST, the membrane was incubated at room temperature for 2 h with horseradish peroxidase-conjugated secondary antibody diluted with TBST, and then visualized using commercial ECL kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. The protein bands were quantified using ImageJ 1.33 software (NIH), and the data were normalized to β-actin.
Statistical analysis
All statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± SE. χ 2 analysis was done to evaluate the significance of differences between the experimental groups. For a single comparison of two groups, Student’s t test was used. A P value of less than 0.05 was considered to be statistically significant.
Results
XIAP is overexpressed in bladder cancer
In this study, we performed IHC staining of XIAP in 35 bladder cancer tissues and corresponding adjacent non-neoplastic tissues. The representative staining picture is shown in Fig. 1. Analysis of the IHC showed that XIAP was significantly upregulated in bladder cancer cases (P < 0.001). When we divided the bladder cancer group into invasive and non-invasive subgroups, IHC scores showed that XIAP was obviously upregulated in the invasive subgroup compared with the non-invasive subgroup (P < 0.01). Together, our data suggest that the overexpression of XIAP may play an important role in bladder cancer initiation and progression.
Embelin inhibits the proliferation of bladder cancer cells
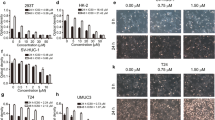
To determine the inhibitory effect of Embelin on bladder cancer cells, CCK-8 assay was performed. When the concentration of Embelin was used respectively at 5, 10, 20, 25, and 35 µmol/l, the survival of both T24 and 5637 cells decreased in a dose-/time-dependent manner (P < 0.05, Fig. 2). Our results showed Embelin had a strong inhibition effect in survival rates of T24 and 5637 cells. In all the following tests, 5, 20, and 35 µmol/l of Embelin was selected to to further investigate the effects of Embelin on bladder cancer cells and explore the related mechanism.
Effect of Embelin used on apoptosis of bladder cancer cells
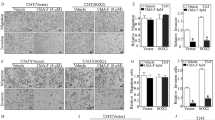
To determine whether Embelin induces apoptosis of T24 and 5637 cells, Annexin V-FITC/PI staining and Western blotting were performed. Annexin V and PI staining results showed that with the increase of Embelin concentration after 24 h, the number of apoptotic cells also increased obviously (P < 0.01, Fig. 3). These data indicated that Embelin could induce more apoptosis on both T24 and 5637 cells. The expression levels of PI3K, Akt, and p-Akt were examined by Western blotting in T24 cells (Fig. 4). The results showed that PI3K and p-Akt decreased significantly after treated by Embelin, which further confirmed that Embelin inhibits cell growth by inducing apoptosis via PI3K/Akt pathway.
Inhibition of migration and invasion of bladder cancer cells by Embelin
We used the transwell assay to verify the Embelin’s effect on migration and invasion of bladder cancer cells in vitro. The results of T24 cells showed that both in invasion assay and migration assay, the number of T24 cells that penetrated through the membrane in the Embelin-treated groups was significantly less than the negative control group. T24 cells in 35 μM group penetrated less cells through the polycarbonate membrane than the 5 μM group (P < 0.05, Fig. 5). These results show a critical role of Embelin in the inhibition on bladder cancer cells migration and invasion.
Discussion
Despite the continued refinement of surgical techniques, the prognosis of bladder cancer has remained disappointingly, with a low 5-year survival rate [10]. Understanding the biology mechanism of bladder cancer progression is important for improving the treatment the disease. XIAP is one of the most potent endogenous inhibitors of the caspases, which expression is elevated in many cancers [11–14]. XIAP inhibits the downstream caspase-3 and -7 by binding them to its BIR2 domain, and the upstream caspase-9 by binding it to its BIR3 domain.
Embelin has been recently noticed as an inhibitor of XIAP to induce cancer cell growth suppression, autophagy, and apoptosis. However, there are few reports about the therapeutic effect of Embelin in bladder cancer cells. In recent years, Embelin has been paid more attention for its anti-cancer properties because it has more selectivity towards cancer cells compared to the normal cells. In the current study, we evaluated the efficacy of Embelin on bladder cancer in vitro. Our research found that that Embelin could inhibit proliferation and induce apoptosis in human bladder cancer cells in a dose-dependent manner, indicating that Embelin may be a potential anti-cancer drug for bladder cancer patients.
In this study, we firstly examined the expression patterns of XIAP in bladder cancer tissues. The expression of XIAP was measured in tissue samples from 35 bladder cancer and paired adjacent non-cancerous tissues (NT) by immunohistochemistry analysis. Results showed that XIAP was significantly upregulated in bladder cancer cases, compared with the adjacent non-tumourous bladder tissues. XIAP was predominantly stained in the cytoplasm, but it was stained diffusely and weakly in the nucleus. This was probably due to the relocation of XIAP from the cytosol to the nucleus.
Previous studies revealed that XIAP has been reported to exert the strongest anti-apoptotic function, which has been linked to its ability to bind to caspase-3, and -9. Embelin also played an important role in the apoptotic effect of cancer cells [15, 16]. It is well believed that Embelin could combine with BIR3 domain of XIAP, and then stop XIAP bonding with caspase-3, caspase-7, and caspase-9 so as to induce apoptosis. Our results showed that the number of apoptotic cells increased obviously with the increase of Embelin concentration, indicating that Embelin could induce apoptosis on both T24 and 5637 cells. However, the exact mechanism of Embelin to induce apoptosis of bladder cancer is unclear.
Previous studies had demonstrated that the expression of PI3K/Akt was elevated in bladder cancer and down-regulation of Akt could reduce XIAP expression levels. Therefore, we wondered if Embelin might induce apoptosis of bladder cancer cells via PI3K/Akt pathway. Our study confirmed that with the increase of concentration of Embelin, the expression levels of PI3K and p-Akt decreased significantly, which further confirmed that Embelin inhibits cell growth by inducing apoptosis via PI3K/Akt pathway.
In conclusion, the present study demonstrated that Embelin inhibits the proliferation and migration, and induces apoptosis of human bladder cancer cells. Embelin may be developed into a novel and potential chemotherapeutic drug for bladder cancer.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–92.
Von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8.
Shirodkar SP, Lokeshwar VB. Potential new urinary markers in the early detection of bladder cancer. Curr Opin Urol. 2009;19(5):488–93.
Garcia-Fernandez M, Kissel H, Brown S, Gorenc T, Schile AJ, Rafii S, et al. Sept4/arts is required for stem cell apoptosis and tumor suppression. Genes Dev. 2010;24(20):2282–93.
Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53(3):153–9.
Joshi R, Kamat JP, Mukherjee T. Free radical scavenging reactions and antioxidant activity of embelin: biochemical and pulse radiolytic studies. Chem Biol Interact. 2007;167(2):125–34.
Chitra M, Devi CS, Sukumar E. Antibacterial activity of embelin. Fitoterapia. 2003;74(4):401–3.
Yang T, Lan J, Huang Q, Chen X, Sun X, Liu X, et al. Embelin sensitizes acute myeloid leukemia cells to TRAIL through XIAP inhibition and NF-κB inactivation. Cell Biochem Biophys. 2015;71(1):291–7.
Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, et al. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10(3):227–39.
Beug ST, LaCasse EC, Korneluk RG. Smac mimetics combined with innate immune stimuli create the perfect cytokine storm to kill tumor cells. Oncoimmunology. 2014;3:e28541.
Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, et al. Xiap restricts tnf- and rip3-dependent cell death and inflammasome activation. Cell Rep. 2014;7(6):1796–808.
Paschall AV, Zimmerman MA, Torres CM, Yang D, Chen MR, Li X, et al. Ceramide targets xiap and ciap1 to sensitize metastatic colon and breast cancer cells to apoptosis induction to suppress tumor progression. BMC Cancer. 2014;14:24.
Moreno-Martinez D, Nomdedeu M, Lara-Castillo MC, Etxabe A, Pratcorona M, Tesi N, et al. Xiap inhibitors induce differentiation and impair clonogenic capacity of acute myeloid leukemia stem cells. Oncotarget. 2014;5(12):4337–46.
Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9(3):459–70.
Li X, Huang T, Jiang G, Gong W, Qian H, Zou C. Synergistic apoptotic effect of crocin and cisplatin on osteosarcoma cells via caspase induced apoptosis. Toxicol Lett. 2013;221(3):197–204.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Fu, X., Pang, X., Qi, H. et al. XIAP inhibitor Embelin inhibits bladder cancer survival and invasion in vitro. Clin Transl Oncol 18, 277–282 (2016). https://doi.org/10.1007/s12094-015-1363-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1363-2