Abstract
Background
The recent immunotherapy treatment on triple-negative breast cancer (TNBC) leads to the breakthrough assignation. In this study, we have tried the new combinations of specific chemo with DC-CIKs immunotherapy to treat those patients.
Patients and methods
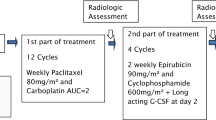
Twenty-three metastatic anthracyclines and taxanes pretreated TNBC younger (mean 41.5 years) patients were initially mobilized with cyclophosphamide (3 g/m2) for the preparation of CD34+ peripheral blood mononuclear cells as the resources for generating DC/CIKs and marrow function supports. All cases were subsequently experienced 2 cycles of chemotherapy with cyclophosphamide 3 g/m2, thiotepa 150 mg/m2, and carboplatin AUC = 6, Q4w. The patients then received 3 infusions of DC-CIKs at the chemo intervals and followed by maintenance therapy with oral cyclophosphamide 50 mg daily. The endpoints were progression-free survival and overall survival.
Results
The partial response rate was 13.0 %, stable and progressive disease rates were 56.5 and 30.4 %, respectively. The median PFS was 13.5 months (95 % confidence interval (CI) 10.1–16.9 months) and OS was 15.2 months (95 % CI 12.5–18.1 months). The most common serious adverse events were neutropenia (100.0 %) and anemia (69.7 %) but without treatment-related mortality.
Conclusion
These data suggested that such combination therapy model be effective and safe for younger metastatic TNBC exposure to previous anthracyclines and taxanes based adjuvant chemotherapy.


Similar content being viewed by others
References
Shastry M, Yardley DA. Updates in the treatment of basal/triple-negative breast cancer. Curr Opin Obstet Gynecol. 2013;25(1):40–8.
Colleoni M, Cole BF, Viale G, Regan MM, Price KN, Maiorano E, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple negative, node negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node negative breast cancer. J Clin Oncol. 2010;28:2966–73.
Cancello G, Bagnardi V, Sangalli C, Montagna E, Dellapasqua S, Sporchia A, et al. Phase II study with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel with metronomic cyclophosphamide as a preoperative treatment of triple-negative breast cancer. Clin Breast Cancer. 2015;. doi:10.1016/j.clbc.2015.03.002.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig. 2011;121(7):2750–67.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34.
Anders CK, Zagar TM, Carey LA. The management of early-stage and metastatic triple-negative breast cancer: a review. Hematol Oncol Clin North Am. 2013;27(4):737–49.
Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann Oncol. 2012;23(Suppl 6:vi):40–5.
Rodler E, Korde L, Gralow J. Current treatment options in triple negative breast cancer. Breast Dis. 2010;32(1–2):99–122.
Gibson J. Anti-PD-L1 for metastatic triple-negative breast cancer. Lancet Oncol. 2015;16(6):e264. doi:10.1016/S1470-2045(15)70208-1.
Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013;5(3):169–81.
Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27(8):1323–33.
Wolff A, Hammond M, Schwartz J, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45.
Yu J, Ren J, Di LJ, Song GH, Zhu YL, Zhang J, et al. Mobilization of peripheral blood stem cells using regimen combining docetaxel with granulocyte colony-stimulating factor. Chin J Cancer Res. 2011;23:49–53.
Jiang HF, Song GH, Che L, Di LJ, Ren J. Metronomic low-dose oral cyclophosphamide plus endocrine therapy as systemic treatment for 23 metastatic breast cancer patients. Chin J Clin Oncol. 2011;38(2):104–7.
Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24(22):3623–8.
Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nolè F, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13(1):73–80.
Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. Am J Roentgenol. 2010;195(2):281–9.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81.
Harris LN, Broadwater G, Lin NU, Miron A, Schnitt SJ, Cowan D, et al. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342. Breast Cancer Res. 2006;8(6):R66.
Arnedos M, Bihan C, Delaloge S, Andre F. Triple-negative breast cancer: are we making headway at least? Ther Adv Med Oncol. 2012;4(4):195–210.
Brouckaert O, Wildiers H, Floris G, Neven P. Update on triple-negative breast cancer: prognosis and management strategies. Int J Womens Health. 2012;4:511–20.
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–9.
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant cisplatin in triple negative breast cancer. J Clin Oncol. 2010;28:1145–53.
O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple negative breast cancer. N Engl J Med. 2011;364:205–14.
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44.
Nanda R, Chow QL, Dees EC, Berger R, Gupta S, Geva R, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. 2014; San Antonio Breast Cancer Symposium (SABCS) ABSTRACT #S1–09.
Ren J, Di L, Song G, Jia J, Zhu Y, Yan Y, et al. Selections of appropriate regimen of high-dose chemotherapy combined with adoptive cellular therapy with dendritic and cytokine-induced killer cells improved progression free and overall survival in patients with metastatic breast cancer: reargument of such contentious therapeutic preferences. Clin Trans Oncol. 2013;15(10):780–8.
Rodenhuis S, Bontenbal M, van Hoesel QG, Smit WM, Nooij MA, Voest EE, et al. Efficacy of high-dose alkylating chemotherapy in HER2/neu-negative breast cancer. Ann Oncol. 2006;17:588–96.
Nitz UA, Gluz O, Herr A, Ting E, Mohrmann S, Frick M, et al. Retrospective analysis of WSG AM01 tandem high dose chemotherapy trial in high risk primary breast cancer: A hypothesis generating study. Proc Am Soc Clin Oncol 2006; 24:665. (Abstr).
Sohn HJ, Kim SH, Lee GW, Kim S, Ahn JH, Kim SB, et al. High-dose chemotherapy of cyclophosphamide, thiotepa and carboplatin (CTCb) followed by autologous stem-cell transplantation as a consolidation for breast cancer patients with 10 or more positive lymph nodes: a 5-year follow-up results. Cancer Res Treat. 2005;37(3):137–42.
Vanderwalde A, Ye W, Frankel P, Asuncion D, Leong L, Luu T, et al. Long-term survival after high-dose chemotherapy followed by peripheral stem cell rescue for high-risk, locally advanced/inflammatory, and metastatic breast cancer. Biol Blood Marrow Transplant. 2012;18(8):1273–80.
Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31(4):490–8.
Lu Z, Jia J, Di L, Song G, Yuan Y, Ma B, et al. DNA methyltransferase inhibitor CDA-2 synergizes with high-dose thiotepa and paclitaxel in killing breast cancer stem cells. Front Biosci. 2011;1:240–9.
Sun H, Jia J, Wang X, Ma B, Di L, Song G, et al. CD44+/CD24− breast cancer cells isolated from MCF-7 cultures exhibit enhanced angiogenic properties. Clin Trans Oncol. 2012;15:46–54.
Radojvic V, Bezak K, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, et al. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2010;59:137–48.
Acknowledgments
We thank and remember the extraordinary courage and fortitude of the patients enrolled in this study who consent to participating this novel therapeutic model. We thank Sherry Gu PhD from Duke Clinical Research Institute of Duke University, Durham, NC, USA, for her assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no any interest conflict of all authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
Beijing Municipal Science and Technology Project (Z151100004015183), Beijing Oncological Leadership Grant (No. 2009-2-16) and Natural Science Foundation of China (No. 81172534).
Rights and permissions
About this article
Cite this article
Wang, X., Ren, J., Zhang, J. et al. Prospective study of cyclophosphamide, thiotepa, carboplatin combined with adoptive DC-CIK followed by metronomic cyclophosphamide therapy as salvage treatment for triple negative metastatic breast cancers patients (aged <45). Clin Transl Oncol 18, 82–87 (2016). https://doi.org/10.1007/s12094-015-1339-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1339-2