Abstract
Background
To determine the effects of endostatin on vascular growth factor receptor 2 (VEGFR2) expression in non-small cell lung cancer (NSCLC) cells and the mechanisms underlying its radiosensitizing effect.
Methods
VEGFR2 mRNA levels were determined in different NSCLC cell lines using qRT-PCR. RT-PCR and Western blot assays were used to assess the expression of mRNA and proteins. The radiosensitivity of the cells was determined by colony-formation assays; and cell apoptosis and cell cycle distribution were determined by flow cytometry.
Results
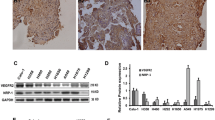
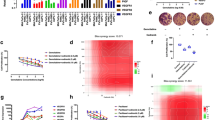
VEGFR2 mRNA levels differed among the five NSCLC cell lines (P < 0.01), with the highest expression in Calu-1 cells and lowest in A549 cells. Endostatin significantly inhibited the growth of Calu-1 cells (P < 0.01) (IC20 = 296.5 μg/ml), and the expression of VEGFR2 and HIF-1α (P < 0.05). Phosphorylation of protein kinase B (Akt), extracellular signal-regulated kinases 1/2 (ERK1/2), and p38 were significantly lower in endostatin-treated cells than control (P < 0.05). Endostatin enhanced the radiosensitivity of Calu-1 cells to SER = 1.38 and induced apoptosis (P < 0.01) and G2/M blockage (P < 0.01). However, endostatin had limited effects on A549 cells. Compared with Calu-1 cells, there was not significantly effects on cell radiosensitivity (SER = 1.09).
Conclusions
Endostatin induces apoptosis and enhances radiosensitivity of the VEGFR2 high-expressing cell line Calu-1, but it has a limited effect on the VEGFR2 low-expressing cell line A549.




Similar content being viewed by others
References
Yeo SG, Kim ES. Efficient approach for determining four-dimensional computed tomography-based internal target volume in stereotactic radiotherapy of lung cancer[J]. Radiat Oncol J. 2013;31(4):247–51.
Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94:1–11.
Langendorff H, Koch R. Studies on biological radioprotection. IX. Effect of SH-blockers on radiosensitivity. Strahlentherapie. 1954;95(4):535–41.
Jiang X-D, Qiao Y, Dai P, Wu J, Song D-A, Li S-Q, et al. Preliminary clinical study of weekly recombinant human endostatin as a hypoxic tumour cell radiosensitiser combined with radiotherapy in the treatment of NSCLC. Clin Transl Oncol. 2012;14(6):465–70.
Jiang X-D, Ding M-H, Qiao Y, Liu Y, Liu L. Study on lung cancer cells expressing VEGFR2 and the impact on the effect of RHES combined with radiotherapy in the treatment of brain metastases. Clin Lung Cancer. 2014;15(2):e23–9.
Donnem T, Al-Shibli K, Andersen S, Al-Saad S, Busund L-T, Bremnes RM. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer. 2010;116:4318–25.
Dhakal HP, Naume B, Synnestvedt M, Borgen E, Kaaresen R, Schlichting E, et al. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptors 1 and 2 in invasive breast carcinoma: prognostic significance and relationship with markers for aggressiveness. Histopathology. 2012;61:350–64.
Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544.
Yang F, Tang X, Riquelme E, Behrens B, Nilsson MB, Giri U, Tang X, et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res. 2011;71(16):5512–21.
Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64(5–6):993–8.
Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49.
Kim Y-M, Hwang S, Kim Y-M, Pyun B-J, Kim T-Y, Lee S-T, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1.[J]. J Biol Chem. 2002;277(31):27872–9.
Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities(and problems)for cancer therapy[J]. Cancer Res. 1998;58(7):1408–16.
Meijer TWH, Kaanders JHAM, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18(20):5585–94.
Agani F, Jiang B-H. Oxygen-independent regulation of HIF-1: novel involvement of PI3 K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–51.
Karapetsas A, Giannakakis A, Pavlaki M, Panayiotidis M, Sandaltzopoulos R, Alex Galanis. Biochemical and molecular analysis of the interaction between ERK2 MAP kinase and hypoxia inducible factor-1α. Int J Biochem Cell Biol. 2011;43(11):1582–90.
Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, et al. The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60(17):4873–80.
Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278(16):14013–9.
Nilsson MB, Zage PE, Zeng L, Xu L, Cascone T, Wu HK, et al. Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors. Oncogene. 2010;29(20):2938–49.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO. 81472792), Research Fund from Ministry of Health (W201210), and the National Natural Science Foundation of Jiangsu Province (BK2012661).
Conflict of interests
We have read and understood Clinical and Translational Oncology’s policy on disclosing conflicts of interest and declare that we have none.
Ethics statement
Our study did not refer to clinical trial and animal trial, so there was no ethics statement.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Liu, Y. Qiao and C. Hu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, L., Qiao, Y., Hu, C. et al. Endostatin exerts radiosensitizing effect in non-small cell lung cancer cells by inhibiting VEGFR2 expression. Clin Transl Oncol 18, 18–26 (2016). https://doi.org/10.1007/s12094-015-1319-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1319-6