Abstract
Purpose
To identify biological markers related to the progression and prognosis of GBC.
Methods
The expressions of pyruvate kinase isoenzyme type M2 (PKM2) and activin A receptor type IC (ACVR 1C) in 46 squamous cell/adenosquamous carcinomas (SC/ASC) and 80 adenocarcinomas (AC) were examined using immunohistochemistry.
Results
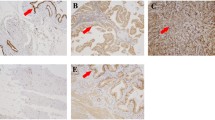
Positive PKM2 and negative ACVR 1C expressions were significantly associated with lymph node metastasis, invasion and TNM stage of SC/ASCs and ACs. Univariate Kaplan–Meier analysis showed that either elevated PKM2 or loss of ACVR 1C expression significantly correlated with shorter average survival times in both SC/ASC and AC patients. Multivariate Cox regression analysis showed that positive PKM2 expression and loss of ACVR 1C expression were poor prognosis biomarkers in both SC/ASC and AC patients.
Conclusions
Our study suggested that PKM2 overexpression is a marker of metastasis, invasion and poor prognosis of GBC. ACVR 1C is a tumor suppressor, and lowered ACVR 1C expression is an important marker for the metastasis, invasion, and prognosis of GBC.



Similar content being viewed by others
References
Jayaraman S, Jarnagin WR. Management of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:331–42.
Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–5.
de Aretxabala X, Roa I, Burgos L, Losada H, Roa JC, Mora J, et al. Gallbladder cancer: an analysis of a series of 139 patients with restricted to the subserosal layer. J Gastrointest Surg. 2006;10:186–92.
Ootani T, Shirai Y, Tsukada K, Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647–52.
Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, et al. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol. 2011;24:1069–78.
Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969–80.
Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–8.
Spoden GA, Mazurek S, Morandell D, Bacher N, Ausserlechner MJ, Jansen-Dürr P, et al. Isotype-specific inhibitors of the glycolytic key regulator pyruvate kinase subtype M2 moderately decelerate tumor cell proliferation. Int J Cancer. 2008;123:312–21.
Jiang K, He B, Lai L, Chen Q, Liu Y, Guo Q, et al. Cyclosporine A inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2. Int J Mol Med. 2012;30:302–8.
Watanabe R. Activin receptor-like kinase and the insulin gene. Vitam Horm. 2011;85:1–27.
Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J. 2008;55:11–21.
Angenete E, Langenskiöld M, Palmgren I, Falk P, Oresland T, Ivarsson ML. Transforming growth factor beta-1 in rectal tumor, mucosa and plasma in relation to radiotherapy and clinical outcome in rectal cancer patients. Int J Colorectal Dis. 2007;22:1331–8.
Joshi A, Cao D. TGF-beta signaling, tumor microenvironment and tumor progression: the butterfly effect. Front Biosci. 2010;15:180–94.
Liu DC, Yang ZL, Jiang S. Identification of PEG10 and TSG101 as carcinogenesis, progression, and poor-prognosis related biomarkers for gallbladder adenocarcinoma. Pathol Oncol Res. 2011;17:859–66.
Kondo M, Dono K, Sakon M, Shimizu J, Nagano H, Nakamori S, et al. Adenosquamous carcinoma of the gallbladder. Hepatogastroenterology. 2002;49:1230–4.
Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007;4:99–124.
Hoda MA, Münzker J, Ghanim B, Schelch K, Klikovits T, Laszlo V, et al. Suppression of activin A signals inhibits growth of malignant pleural mesothelioma cells. Br J Cancer. 2012;107:1978–86.
Conflict of interest
All authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Yang, Z., Zou, Q. et al. PKM2 and ACVR 1C are prognostic markers for poor prognosis of gallbladder cancer. Clin Transl Oncol 16, 200–207 (2014). https://doi.org/10.1007/s12094-013-1063-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1063-8