Abstract
Purpose
To compare the genoprotective and radioprotective effect of carnosol (COL) against damage induced by ionizing radiation with similar effects produced by different antioxidant compounds.
Methods
The genoprotective effect was studied by means of the micronucleus test for antimutagenic activity in which the reduction in the frequency of micronuclei was evaluated in cytokinesis-blocked cells of human lymphocytes. The radioprotective effects were studied by cell viability test (MTT) in PNT2 (normal prostate) and B16F10 (melanoma) cell lines when they were administered before exposure to different X-ray doses (4, 6, 8, 10 and 0 Gy).
Results
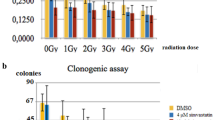
Carnosol shows a significant genoprotective capacity (p < 0.001) against radiation with a protection factor of 50 %, and a dose-reduction factor of 4.3. Cell survival obtained with COL administered before exposure to 10 Gy of X-rays showed a protection factor of 55.1 %, eliminating 39 % of radiation-induced cell death in normal epithelial cells of prostate (PNT2) (p < 0.001). However, in the melanoma cell lines (B16F10) assayed, COL acted not as a radioprotector, but as a sensitizing agent increasing the cellular death by 34 % (p < 0.01) and producing an enhancement ratio of 2.12.
Conclusions
Carnosol may be developed as a radioprotective agent in the non-tumoral cells. However, in the B10F16 melanoma cells, melanogenesis is activated by COL leading to redistribution of the enzymatic balances of glutathione and cysteine-lyase production, which could compromise the intracellular redox defence system. This effect appears as an increase in the capacity of ionizing radiation-induced damage, and thus exhibits a paradoxical protective effect of COL on melanoma cells.





Similar content being viewed by others
References
Del Baño MJ, Lorente J, Castillo J, Benavente-García O, Del Río JA et al (2003) Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J Agric Food Chem 51:4247–4253
Johnson JJ (2011) Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett 305:1–7
Johnson JJ, Syed DN, Suh Y, Heren CR, Saleem M, Siddiqui IA, Mukhtar H (2010) Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev Res (Phila) 3:1112–1123
Alcaraz M, Acevedo C, Castillo J, Benavente-Garcia O, Armero D et al (2009) Liposoluble antioxidants provide an effective radioprotective barrier. Br J Radiol 82:605–609
Castillo J, Benavente-Garcia O, Lorente J, Alcaraz M, Redondo A et al (2000) Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (procyanidins) from grape seeds (Vitis vinifera): comparative study versus other phenolic and organic compounds. J Agric Food Chem 48:1738–1745
Castillo J, Benavente-Garcia O, Del Bano MJ, Lorente J, Alcaraz M, Dato MJ (2001) Radioprotective effects against chromosomal damage induced in human lymphocytes by gamma-rays as a function of polymerization grade of grape seed extracts. J Med Food 4:117–123
IAEA (2011) Cytogenetic dosimetry: applications in preparedness for and response to radiation emergencies. IAEA, Vienna
Hall EJ (1978) Radiobiology for the radiologist, 2nd edn. Harper & Row, Philadelphia
Alcaraz M, Armero D, Martinez-Beneyto Y, Castillo J, Benavente-Garcia O et al (2011) Chemical genoprotection: reducing biological damage to as low as reasonably achievable levels. Dentomaxillofac Radiol 40:310–314
Sarma L, Kesavan PC (1993) Protective effects of vitamins C and E against gamma-ray-induced chromosomal damage in mouse. Int J Radiat Biol 63:759–764
Sánchez-Campillo M, Gabaldon JA, Castillo J, Benavente-Garcia O, Del Bano MJ et al (2009) Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol 47:386–392
Benavente-Garcia O, Castillo J, Lorente J, Alcaraz M (2002) Radioprotective effects in vivo of phenolics extracted from Olea europaea L. leaves against X-ray-induced chromosomal damage: comparative study versus several flavonoids and sulfur-containing compounds. J Med Food 3:125–135
Del Baño MJ, Castillo J, Benavente-Garcia O, Lorente J, Martin-Gil R et al (2006) Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by gamma-rays. J Agric Food Chem 54:2064–2068
Castillo J, Alcaraz M, Benavente-García O (2010) Antioxidant and radioprotective effects of olive leaf extract. In: Preedy VR, Watson RR (eds) Olives and Olive oil in health and disease prevention. Academic Press, Oxford, pp 951–958
Martinez Conesa C, Vicente Ortega V, Yanez Gascon MJ, Alcaraz-Banos M, Canteras Jordana M et al (2005) Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J Agric Food Chem 53:6791–6797
Yañez J, Vicente V, Alcaraz M, Castillo J, Benavente-Garcia O et al (2004) Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: relationship between structure and activity. Nutr Cancer 49:191–199
Benavente-García O, Castillo J, Lorente J, Alcaraz M, Yáñez J, Martínez C (2005) Antiproliferative activity of several phenolic compounds against melanoma cell lines. Agro Food Ind Hi Tech 16:30–34
Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F (2010) Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr 65:158–163
Kim HJ, Cho YD, Leem KH, Lee DN, Kim EH et al (2006) Effects of Ephedrae herba on melanogenesis and gene expression profiles using cDNA microarray in B16 melanocytes. Phytothe Res 20:748–754
Ohguchi K, Akao Y, Nozawa Y (2006) Stimulation of melanogenesis by the citrus flavonoid naringenin in mouse B16 melanoma cells. Biosci Biotechnol Biochem 70:1499–1501
Kudugunti SK, Vad NM, Ekogbo E, Moridani MY (2011) Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16–F0 melanoma tumor bearing C57BL/6 mice. Invest New Drugs 29:52–62
Lee J, Kim YS, Park D (2007) Rosmarinic acid induces melanogenesis through protein kinase A activation signaling. Biochem Pharmacol 74:960–968
Panich U, Onkoksoong T, Limsaengurai S, Akarasereenont P, Wongkajornsilp A (2012) UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: the protective effect of gallic acid. J Photochem Photobiol B 108:16–22
Panich U, Tangsupa-a-nan V, Onkoksoong T, Kongtaphan K, Kasetsinsombat K, Akarasereenont P, Wongkajornsilp A (2011) Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch Pharm Res 34(5):811–820
Kwee JK, Mitidieri E, Affonso OR (1991) Lowered superoxide dismutase in highly metastatic B16 melanoma cells. Cancer Lett 57:199–202
Acknowledgments
This report was supported by a grant from the National Spanish R&D Programme CENIT (Acronym:SENIFOOD); D.G. Achel was able to take part in this study because of a sponsored fellowship from IAEA and A. Olivares thanks to a fellowship from the Seneca Foundation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This report was supported by a grant from the National Spanish R&D Programme CENIT of the Spanish Ministry of Science and Technology denominated “Industrial research diets and food with specific features for the elderly” (Acronym: SENIFOOD); DG. Achel was able to take part in this study because of a sponsored fellowship (GHA/0021) from International Atomic Energy Agency (IAEA) and A. Olivares thanks to a grant from the Seneca Foundation (Coordination Research Centre of the Region of Murcia, Spain).
Rights and permissions
About this article
Cite this article
Alcaraz, M., Achel, D.G., Olivares, A. et al. Carnosol, radiation and melanoma: a translational possibility. Clin Transl Oncol 15, 712–719 (2013). https://doi.org/10.1007/s12094-012-0994-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0994-9