Abstract
Purpose
Immunotherapy (IL-2 and INF-α) was the treatment of choice for advanced renal cell carcinoma (RCC) until antiangiogenic therapy with tyrosin kinase inhibitors was developed in the early 2000s. This clinical trial explored the efficacy and toxicity of sequential treatment of IL-2 plus INF-α followed by sorafenib.
Methods
Eligibility criteria included measurable, non-resectable, histologically confirmed predominantly clear cell RCC, no prior systemic treatment, and ECOG PS 0–2. The treatment regimen was a 6-week cycle of subcutaneous IL-2 at 9 × 106 IU on days 1–6 of weeks 1, 2, 4 and 5 plus s.c. INF-α at 6 × 106 IU on days 1, 3 and 5 of weeks 1–6. Responders received 6 additional weeks of this regimen. All patients received oral sorafenib (400 mg bid) after immunotherapy until disease progression. The primary endpoint was progression-free survival.
Results
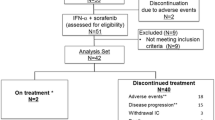
Forty-one patients were enrolled, median age 57 years. ECOG was 0/1 in 17/20 patients, 35 patients had prior nephrectomy and 18 patients pure clear cell cancer. Median PFS was 7.4 months (95 % CI 6.5–13.1) and OS was 16.6 months (95 % CI not reached). In 36 patients evaluable for response, ORR was 44.4 % and control rate was 94.4 %. Most adverse events (AEs) were Grade 1 or 2 toxicities (84.7 %). During immunotherapy the most common AEs were pyrexia (82.9 %), asthenia (56.1 %) and anorexia (46.3 %), whereas during sorafenib were diarrhoea (48.8 %) and hand–foot syndrome (46.3 %).
Conclusions
A sequential regimen of IL-2 and INF-α followed by sorafenib showed effectiveness and manageable toxicity in patients with advanced RCC.




Similar content being viewed by others
References
Bukowski R (1999) Immunotherapy in renal cell carcinoma. Oncology 13:801–813
Coppin C, Porzsolt F, Awa A et al (2005) Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev 25:CD001425
Negrier S, Maral J, Drevon M et al (2000) Long-term follow-up of patients with metastatic renal cell carcinoma treated with intravenous recombinant interleukin-2 in Europe. Cancer J Sci Am 6(Suppl 1):S93–S98
Escudier B, Szczylik C, Hutson TE et al (2009) Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27:1280–1289
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27:3584–3590
Yang JC, Haworth L, Sherry RM et al (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427–434
Rini BI, Halabi S, Rosenberg JE et al (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26:5422–5428
Escudier B, Bellmunt J, Négrier S et al (2010) Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 1:2144–2150
Motzer RJ, Bacik J, Schwartz LH et al (2004) Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22:454–463
Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. March 31, 2003 (http://ctep.cancer.gov), Publish Date: August 9, 2006
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Motzer RJ, Bacik J, Murphy BA et al (2006) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289–296
Escudier B, Pluzanska A, Koralewski P et al (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370:2103–2111
Miyake H, Kusuda Y, Harada KI, Sakai I, Fujisawa M (2011) Third-line sunitinib following sequential use of cytokine therapy and sorafenib in Japanese patients with metastatic renal cell carcinoma. Int J Clin Oncol 18 (published ahead of printing)
Ryan CW, Goldman BH, Lara PM et al (2007) Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J Clin Oncol 25:3296–3301
Egberts F, Gutzmer R, Ugurel S et al (2011) Sorafenib and pegylated interferon-α2b in advanced metastatic melanoma: a multicenter phase II DeCOG trial. Ann Oncol 22:1667–1674
Procopio G, Verzoni E, Bracarda S et al (2011) Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. Br J Cancer 104:1256–1261
Negrier S, Perol D, Ravaud A et al (2007) Medroxyprogesterone, interferon alpha 2a, interleukin-2 or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis. Results of a randomized controlled trial. Cancer 110:2468–2477
Escudier B, Porta C, Bono P et al (2012) Patient preference between pazopanib (Paz) and sunitinib (Sun): results of a randomized double-blind, placebo-controlled, cross-over study in patients with metastatic renal cell carcinoma (mRCC)—PISCES study. Clin Oncol 30, (Suppl; abstr CRA4502)
Motzer RJ, Nosov D, Eisen T et al (2012). Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, openlabel, multicenter trial. J Clin Oncol 30(Suppl; abstr 4501)
Rini BI, Szczylik C, Tannir P et al (2011) AMG 386 in combination with sorafenib in patients (pts) with metastatic renal cell cancer (mRCC): a randomized, double-blind, placebo-controlled, phase II study. J Clin Oncol 29 (Suppl 7, abstr 309)
Escudier B, Eisen T, Stadler WM et al (2007) (2011) Sorafenib in advanced clear cell. Renal-cell carcinoma. N Engl J Med 356:125–134
Escudier B, Perol D, Ferlay C et al (2010) Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma? Results of the randomised Torava phase II trial. J Clin Oncol 28:155 (abstr 4516)
Wong MKK (2011) The current role of immunotherapy for renal cell carcinoma in the era of targeted therapeutics. Curr Oncol Rep 10:259–263
Acknowledgments
We are grateful to the patients who participated in this trial. Funding for this study was provided by Bayer Hispania S.L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maroto, J.P., del Muro, X.G., Mellado, B. et al. Phase II trial of sequential subcutaneous interleukin-2 plus interferon alpha followed by sorafenib in renal cell carcinoma (RCC). Clin Transl Oncol 15, 698–704 (2013). https://doi.org/10.1007/s12094-012-0991-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0991-z