Abstract
Introduction
Concurrent chemotherapy and radiotherapy is recommended for the treatment of locally advanced unresectable head and neck (H&N) cancer.
Objective
The primary purpose of the Phase I part of the study was to determine the maximum tolerated dose (MTD), dose-limiting toxicity (DLT) and recommended dose (RD) of docetaxel with hyperfractionation radiotherapy. The primary objective of the Phase II part was to determine the response rate to the RD of treatment and, secondarily, to assess the toxicity of the schedule, time to progression, duration of response and overall survival (OS).
Materials and methods
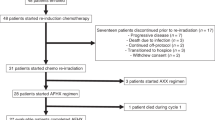
Patients (n=9 in Phase I; n=19 in Phase II) had unresectable H&N cancer. The starting docetaxel dose was 20 mg/m2 plus hyperfractionated radiotherapy. Ramping of docetaxel was 5 mg/m2 if MTD was not reached.
Results
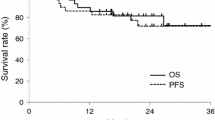
MTD of docetaxel was 20 mg/m2. Limiting toxicities were grade 4 pneumonia and grade 4 mucositis. The RD was 15 mg/m2. Phase II initial response was 76% (CR=18%; PR=9%); updated response was 89% (CR=59%; PR=29%). The median progression-free survival was 7.8 months (95%CI: 0–22.3) and the median OS was 15.1 months (95%CI: 0–35.9). Grade 3–4 toxicities included mucositis (91%), pneumonia (27%) and fatigue (27%). There were 5 toxic deaths (2 from intestinal perforation, 3 from pneumonia).
Conclusions
Weekly docetaxel+hyperfractionation radiotherapy is active but with high toxicity rates and, hence, this treatment regimen would be difficult to justify.
Similar content being viewed by others
References
Mendenhall WM, Riggs ChE, Cassisi N (2005) Treatment of head and neck cancers. In: De Vita VT, Hellman S, Rosenberg SA (eds) Cancer principles and practice of oncology, 8th edn. Lippincott Williams and Wilkins, Philadelphia, pp 809–876
Pignon JP, Bourhis J, Domenge C, Designé L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355:949–955
Bourhis J, Overgaard J, Audry H et al; Metaanalysis of Radiotherapy in Carcinomas of Head and Neck (MARCH) Collaborative Group (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368:843–854
Budach W, Hehr T, Budach V et al (2006) A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 6:28
Adelstein DJ, Li Y, Adams GL et al (2003) An intergroup Phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Schrijvers D, Vermorken JB (2005) Taxanes in the treatment of head and neck cancer. Curr Opin Oncol 17:218–224
Schrijvers D, Van Herpen C, Kerger J et al (2004) Docetaxel, cisplatin and 5-fluorouracil in patients with locally advanced unresectable head and neck cancer: a Phase I–II feasibility study. Ann Oncol 15:638–845
Pignon JP, Syz N, Posner M et al (2004) Adjusting for patient selection suggests the addition of docetaxel to 5-fluorouracil-cisplatin induction therapy may offer survival benefit in squamous cell cancer of the head and neck. Anticancer Drugs 15:331–340
Ringel I, Horwitz SB (1991) Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst 83:288–291
Tishler RB, Norris CM Jr, Colevas AD et al (2002) A Phase I/II trial of concurrent docetaxel and radiation after induction chemotherapy in patients with poor prognosis squamous cell carcinoma of the head and neck. Cancer 95:1472–1481
Matsumoto F, Karasawa K, Itoh S et al (2006) Concurrent weekly docetaxel and hyperfractionated radiotherapy for advanced head and neck cancer. Anticancer Res 26:3781–3786
Biete Solà A, Marruecos Querol J, Calvo Manuel FA et al (2007) Phase II trial: concurrent radio-chemotherapy with weekly docetaxel for advanced squamous cell carcinoma of head and neck. Clin Transl Oncol 9:244–250
Tsao AS, Garden AS, Kies MS et al (2006) Phase I/II study of docetaxel, cisplatin, and concomitant boost radiation for locally advanced squamous cell cancer of the head and neck. J Clin Oncol 24:4163–4169
Allal AS, Zwahlen D, Becker M et al (2006) Phase I trial of concomitant hyperfractionated radiotherapy with docetaxel and cisplatin for locally advanced head and neck cancer. Cancer J 12:63–68
Schwartz DL, Montgomery RB, Yueh B et al (2005) Phase I and initial phase II results from a trial investigating weekly docetaxel and carboplatin given neoadjuvantly and then concurrently with concomitant boost radiotherapy for locally advanced squamous cell carcinoma of the head and neck. Cancer 103:2534–2543
Choy H, Rodriguez FF, Koester S et al (1993) Investigation of taxol as a potential radiation sensitizer. Cancer 71:3774–3778
Ajani JA, Welch SR, Raber MN et al (1990) Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 8:147–159
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346
Simon R (1989) Optimal two-stage designs for Phase II clinical trials. Control Clin Trials 10:1–10
WHO (1979) WHO handbook for reporting results of cancer treatment. WHO, Geneva
Katori H, Tsukuda M, Watai K (2007) Comparison of hyperfractionation and conventional fractionation radiotherapy with concurrent docetaxel, cisplatin and 5-fluorouracil (TPF) chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). Cancer Chemother Pharmacol 60:399–406
Carter DL, Asmar L, Barrera D et al (2008) Favorable survival observed after carboplatin, paclitaxel, and concurrent accelerated hyperfractionated radiotherapy for treatment of locally advanced head and neck carcinoma. Invest New Drugs 26:473–481
Cmelak AJ, Murphy BA, Burkey B et al (2007) Taxane-based chemoirradiation for organ preservation with locally advanced head and neck cancer: results of a Phase II multi-institutional trial. Head Neck 29:315–324
Cmelak AJ, Li S, Goldwasser MA et al (2007) Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol 25:3971–3977
Hitt R, López-Pousa A, Martínez-Trufero J et al (2005) Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 23:8636–864
Zorat PL, Paccagnella A, Cavaniglia G et al (2004) Randomized Phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst 96:1714–1717
Paccagnella A, Ghi MG, Loreggian L et al; for the Gruppo di Studio Tumori della Testa e del Collo XRP 6976 F/2501 Study (2010) Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol 21:1515–1522
Bourhis J, Rivera F, Mesia R et al (2006) Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 24:2866–2872
Author information
Authors and Affiliations
Corresponding author
Additional information
The affiliations are listed at the end of the article
Rights and permissions
About this article
Cite this article
Barnadas, A., Mesía, R., Majem, M. et al. Phase I/II docetaxel plus concurrent hyperfractionated radiotherapy in locally advanced unresectable head and neck cancer (TAX.ES1.102 study). Clin Transl Oncol 13, 254–260 (2011). https://doi.org/10.1007/s12094-011-0650-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-011-0650-9