Abstract
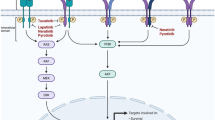
Breast cancer is a heterogeneous disease characterised by a dysregulation of multiple pathways related to cell differentiation, cell cycle control, apoptosis, angiogenesis and development of metastasis. Acting against these pathways provides therapeutic targets for new targeted biologic therapies, which, in the future, might constitute a key for fighting cancer. The development of molecular technology in recent years has allowed a further comprehension of these mutations and dysregulated pathways leading to oncogenesis. New targeted biologic therapies will block essential functions of cancer cells and tumour stroma. A growing number of therapy options, alone or in combination with background treatments (chemotherapy, hormone therapy, radiotherapy), will allow oncologists a better adaptation of treatment to patients and disease characteristics. Examples of approved targeted agents in breast cancer include agents targeting the human epidermal growth factor receptor 2 (HER2), such as trastuzumab, lapatinib and the anti-VEGF bevacizumab. In addition, there are other therapy classes under evaluation, including novel antiEGFR or antiHER2 therapies; agents fighting other tyrosine kinases, including the Src and the insulin-like growth factor receptor; agents interfering critically relevant pathways, such as PI3K/AKT/mTOR inhibitors; and agents promoting apoptosis, such as PARP inhibitors (for particular breast cancer subtypes, such as basal-like, or breast cancer with BRCA mutations) and others. The better selectivity against malignant cells of these therapies, when compared to conventional chemotherapy, gives, a priori, at least two advantages to biologic treatments: fewer side effects and a more individualised treatment of cancer depending on the tumour’s molecular characteristics. The ability to identify patients’ subgroups and response predicting factors will be crucial in obtaining the greatest benefit with minimal toxicity levels. Unsolved questions remain, such as appropriate patient selection based on the expression of the therapeutic target in the tumour, the study of the efficacy of the drug in not so extensively pretreated populations and with a greater chance of response, the use of new pharmacodynamic models to help to define new response predicting factors for a specific new biologic therapy, the combined and rational use of different biologic therapies having different molecular targets and fighting the same target through a complementary mechanism of action that might improve clinical efficacy.
Similar content being viewed by others
References
Ferlay J, Bray F, Pisani O, Parkin DM (2004) GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide, Version 2.0. IARC Cancer Base, No. 5
Slamon DJ, Godolphin W, Jones LA et al (1989) Studies of the HER-2/neu proto-oncogen in human breast and ovarian cancer. Science 244:707–712
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that over-expresses HER2. N Engl Med 344:783–792
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor 2-positive metastatic breast cancer administered as first line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
Vogel CL, Burris HA, Limentani S et al (2009) A phase II study of trastuzumab-DM1 (T-DM1), a HER2 antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, Orlando, FL. Abstract 1017
Krop IE, Burris HA, Rugo H et al (2009) Quantitative assessment of HER2 status and correlation with efficacy for patients (pts) with metastatic breast cancer (MBC) in a phase II study of trastuzumab-DM1 (T-DM1). Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, Orlando, FL, Abstract 1003
De Laurentiis M, Arpino G, Massarelli E et al (2005) A meta-analysis on the interaction between HER2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11:4741–4748
Dowsett M, Allred C, Knox J et al (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER2) status with recurrence in the Arimidex, Tamoxifen, alone or in combination trial. J Clin Oncol 26:1059–1065
Rasmussen BB, Regan MM, Lykkesfeldt AE et al (2008) Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1-98 randomized trial. Lancet Oncol 9:4–5
Kaufman B, Mackey J, Clemens M et al (2006) Trastuzumab plus anastrozole prolongs progression-free survival in postmenopausal women with Her-2 positive, hormone-dependent metastatic breast cancer. Ann Oncol 17[Suppl 9]:Abstract LBA2
Tripathy D, Slamon DJ, Cobleigh M et al (2004) Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol 22:1063–1070
Von Minckwitz G, Zielinski C, Maarteense E et al (2008) Capecitabine vs. capecitabine+ trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: the TBP phase III study (GBG 26/BIG 3-05). J Clin Oncol 26[Suppl]:abstr 1025
Cardoso F, Piccart MJ, Durbecq V et al (2002) Resistance to trastuzumab: a necessary evil or a temporary challenge? Clin Breast Cancer 3:247–257
Bendell J, Domchek S, Burstein H et al (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972–2977
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Slamon D, Eiermann W, Robert N et al (2005) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER-2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat 94:S5
Joensuu H, Kellokumpu-Lehtinen P-L, Bono P et al (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354:809–820
Kaklami V, O’Regan RM (2004) New targeted therapies in breast cancer. Semin Oncol 31[Suppl 4]:420–425
Baselga J, Trigo JM, Bournis J et al (2005) Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 23:5568–5577
Carey L, Rugo H, Marcom P et al (2008) TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal like) breast cancer. J Clin Oncol 26[Suppl]:abstract 1009
O’shaughnessy J, Weckstein D, Vukelja S et al (2007) Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. Breast Cancer Res Treat 106[Suppl 1]:S32
Burris HA (2004) Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist 9[Suppl 3]:10–15
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2 positive advanced breast cancer. N Engl J Med 355:2733–2743
O’shaughnessy J, Blackwell KL, Burstein H et al (2008) A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy. J Clin Oncol 26[Suppl]:abstr 1015
Cortes J, Baselga J, Petrella K et al (2009) Pertuzumab monotherapy following trastuzumab-based treatment: activity and tolerability in patients with advanced HER-2 positive Breast Cancer. Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, Orlando, FL. Abstract 1022
Swaby R, Blackwell K, Jiang Z et al (2009) Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a phase I/II study. Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, Orlando, FL, Abstract 1004
Allen LF, Eiseman IA, Fry DW et al (2003) CI-1033 an irreversible pan-erbB receptor inhibitor and its potential application for the treatment of breast cancer. Semin Oncol 30[5 Suppl 16]:65–78
Raymond E, Alexandre J, Faivre S et al (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 2:2336–2347
Yao J, Phan A, Chang D et al (2006) Phase II study of RAD001 (everolimus) and depot octreotide (Sandostatin LAR) in patients with advanced low grade neuroendocrine carcinoma (LGNET), ASCO Annual Meeting Proceedings Part I. J Clin Oncol 24[Suppl]:4042
Carpenter JT, Roche H, Campone M et al (2005) Randomized 3-arm, phase 2 study of temsirolimus (CCI-779) in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. Proc Am Soc Clin Oncol 23:A564
Baselga J, Semiglazov V, van Dam P et al (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2630–2637
Huang F, Reeves K, Han X et al (2007) Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res 67:2226–2238
Finn RS, Bengala C, Ibrahim N et al (2008) Phase II trial of dasatinib in triple-negative breast cancer: results of study CA180059. Program and abstracts of the 31st Annual San Antonio Breast Cancer Symposium, December 10–14, 2008, San Antonio, TX, Abstract 3118
Modi S, Stopeck A, Gordon MS et al (2006) Phase I trial of KOS-953, a heat shock protein 90 inhibitor, and trastuzumab. J Clin Oncol 24:501 (meeting abstract)
Miller K, Rosen L, Modi S et al (2007) Phase I trial of alvespimycin and trastuzumab, ASCO Annual Meeting Proceedings 18s June 20 Supplement. J Clin Oncol 25:1115
Cobleigh MA, Langmuir VK, Sledge GW et al (2003) A phase I/II dose escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 30:117–124
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Miller KD, Wang M, Gralow J et al (2007) Paclitexel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Miles D, Chan A, Romieu G et al (2008) Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. J Clin Oncol 26[Suppl]:abstr LBA1011
Robert NJ, Dieras V, Glaspy J et al (2009) RIBBON-1: randomized double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC). Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, 2009, Orlando, FL, Abstract 1005
Burstein HJ, Elias AD, Rugo HS et al (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an antracycline and a taxane. J Clin Oncol 26:1810–1816
Moreno-Aspitia A, Morton RF, Hillman DW et al (2008) Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines and taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol 27:11–15
Ashworth A (2008) A synthetic lethal therapeutic approach: Poly (ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26:3785–3790
Fong P, Spicer J, Reade S et al (2006) Phase I pharmacokinetic and pharmacodynamic evaluation of a small molecule inhibitor of Poly ADPRibose Polymerase (PARP), KU-00594336 (ku) in patients with advanced tumours, ASCO Annual Meeting Proceedings, Part I, 18S (June 20 Supplement). J Clin Oncol 24:3022
O’shaughnessy J, Osborne C, Pippen J et al (2009) Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): results of a randomized phase II trial. Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, Orlando, FL, Abstract 3
Tutt A, Robson M, Garber JE et al (2009) Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer. Program and abstracts of the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, Orlando, FL, Abstract CRA501
Farmer H, McCabe N, Lord CJ et al (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917–921
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by an unrestricted educational grant from Pfizer
Rights and permissions
About this article
Cite this article
Sánchez-Muñoz, A., Pérez-Ruiz, E., Jiménez, B. et al. Targeted therapy of metastatic breast cancer. Clin Transl Oncol 11, 643–650 (2009). https://doi.org/10.1007/s12094-009-0419-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-009-0419-6