Abstract
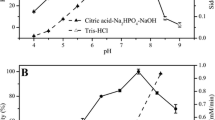
A novel alcohol dehydrogenase from Bartonella apis (BaADH) was heterologous expressed in Escherichia coli. Its biochemical properties were investigated and used to catalyze the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate ((S)-CHBE), which is a chiral intermediate of the cholesterol-lowering drug atorvastatin. The purified recombinant BaADH displayed 182.4 U/mg of the specific activity using ethyl 4-chloroacetoacetate as substrate under the conditions of 50 °C in pH 7.0 Tris–HCl buffer. It was stable in storage buffers of pH 7 to 9 and retains up to 96.7% of the initial activity after 24 h. The Km and Vmax values of BaADH were 0.11 mM and 190.4 μmol min−1 mg−1, respectively. Synthesis of (S)-CHBE catalyzed by BaADH was performed with a cofactor regeneration system using a glucose dehydrogenase, and a conversion of 94.9% can be achieved after 1 h reaction. Homology modeling and substrate docking revealed that a typical catalytic triad is in contact with local water molecules to form a catalytic system. The results indicated this ADH could contribute to the further enzymatic synthesis of (S)-CHBE.






Similar content being viewed by others
References
Kallberg Y, Oppermann U, Jörnvall H, Persson B (2010) Short-chain dehydrogenase/reductase (SDR) relationships: a large family with eight clusters common to human, animal, and plant genomes. Protein Sci 11:636–641. https://doi.org/10.1110/ps.26902
You ZY, Liu ZQ, Zheng YG (2013) Characterization of a newly synthesized carbonyl reductase and construction of a biocatalytic process for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with high space-time yield. Appl Microbiol Biotechnol 98:1671–1680. https://doi.org/10.1007/s00253-013-5042-3
Wu J, Pai CC, Kwok WH, Guo RW, Au-Yeung TTL, Yeung CH, Chan ASC (2003) Studies on the rhodium- and ruthenium-catalyzed asymmetric hydrogenation of α-dehydroamino acids using a family of chiral dipyridylphosphine ligand (P-Phos). Tetrahedron Asymmetry 14:987–992. https://doi.org/10.1016/S0957-4166(03)00098-3
An M, Cai P, Yan M, Hao N, Wang S, Liu H, Li Y, Xu L (2012) A novel reductase from Candida albicans for the production of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 76:1210–1212. https://doi.org/10.1271/bbb.120048
Inoue K, Makino Y, Dairi T, Itoh M (2006) Gene cloning and expression of Leifsonia alcohol dehydrogenase (LSADH) involved in asymmetric hydrogen-transfer bioreduction to produce (R)-form chiral alcohols. J Agric Chem Soc Jpn 70:418–426
Zhang Y, Wang H, Chen L, Wu K, Xie J, Wei D (2016) Efficient production of ethyl (R)-4-chloro-3-hydroxybutanoate by a novel alcohol dehydrogenase from Lactobacillus curieae S1L19. J Mol Catal B Enzym 134:51–60. https://doi.org/10.1016/j.molcatb.2016.09.010
Kumar P, Patel SKS, Lee JK, Kalia VC (2013) Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv 31:1543–1561. https://doi.org/10.1016/j.biotechadv.2013.08.007
Liu CH, Li XQ, Jiang XP, Zhuang MY, Zhang JX, Bao CH, Zhang YW (2016) Preparation of functionalized graphene oxide nanocomposites for covalent immobilization of NADH oxidase. Nanosci Nanotechnol Lett 8:164–167. https://doi.org/10.1166/nnl.2016.2102
Kumar P, Ray S, Patel SKS, Lee JK, Kalia VC (2015) Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int J Biol Macromol 78:9–16. https://doi.org/10.1016/j.ijbiomac.2015.03.046
Gao H, Kim IW, Choi JH, Khera E, Wen F, Lee JK (2015) Repeated production of L-xylulose by an immobilized whole-cell biocatalyst harboring L-arabinitol dehydrogenase coupled with an NAD(+) regeneration system. Biochem Eng J 96:23–28. https://doi.org/10.1016/j.bej.2014.12.017
Moon HJ, Tiwari MK, Singh R, Kang YC, Lee JK (2012) Molecular determinants of the cofactor specificity of ribitol dehydrogenase, a short-chain dehydrogenase/reductase. Appl Environ Microbiol 78:3079–3086. https://doi.org/10.1128/AEM.07751-11
Gao H, Khera E, Lee JK, Wen F (2016) Immobilization of multi-biocatalysts in alginate beads for cofactor regeneration and improved reusability. JoVE J Vis Exp 110:e53944. https://doi.org/10.3791/53944
Ray S, Kalia VC (2017) Microbial cometabolism and polyhydroxyalkanoate co-polymers. Indian J Microbiol 57:39–47. https://doi.org/10.1007/s12088-016-0622-4
Ye Q, Ouyang PK, Ying HJ (2011) A review—biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives. Appl Microbiol Biotechnol 89:513–522. https://doi.org/10.1007/s00253-010-2942-3
Morikawa S, Nakai T, Yasohara Y, Nanba H, Kizaki N, Hasegawa J (2005) Highly active mutants of carbonyl reductase S1 with inverted coenzyme specificity and production of optically active alcohols. Biosci Biotechnol Biochem 69:544–552. https://doi.org/10.1271/bbb.69.544
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Zhang YW, Jeya M, Lee JK (2010) L-Ribulose production by an Escherichia coli harboring l-arabinose isomerase from Bacillus licheniformis. Appl Microbiol Biotechnol 87:1993–1999. https://doi.org/10.1007/s00253-010-2600-9
Liu ZQ, Ye JJ, Shen ZY, Hong HB, Yan JB, Lin Y, Chen ZX, Zheng YG, Shen YC (2015) Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl Microbiol Biotechnol 99:2119–2129. https://doi.org/10.1007/s00253-014-6245-y
Xu Z, Jing K, Liu Y, Cen P (2007) High-level expression of recombinant glucose dehydrogenase and its application in NADPH regeneration. J Ind Microbiol Biotechnol 34:83–90. https://doi.org/10.1007/s10295-006-0168-2
Qi Y, Hou C, Lan M, Ming Y, Yan W, Qinting H, Jian L, Lin X, Yong C, Jian X (2010) Biosynthesis of (S)-4-chloro-3-hydroxybutanoate ethyl using Escherichia coli co-expressing a novel NADH-dependent carbonyl reductase and a glucose dehydrogenase. Bioresour Technol 101:8911–8914. https://doi.org/10.1016/j.biortech.2010.06.098
Hiroaki Y, Kazuya M, Norihiro K, Akinobu M, Nobuyoshi E, Yoshinori K (2004) A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. J Agric Chem Soc Jpn 68:638–649
Gao J, Wang AR, Jiang XP, Zhang JX, Zhang YW (2018) Preparation of expoxy-functionalized magnetic nanoparticles for immobilization of glycerol dehydrogenase. J Nanosci Nanotechnol 18:4852–4857. https://doi.org/10.1166/jnn.2018.15266
Cao H, Mi L, Ye Q (2011) Purification and characterization of a novel NADH-dependent carbonyl reductase from Pichia stipitis involved in biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresour Technol 102:1733–1739. https://doi.org/10.1016/j.biortech.2010.08.072
Nie Y, Xu Y, Hu QS, Xiao R (2009) A new strategy to improve the efficiency and sustainability of Candida parapsilosis catalyzing deracemization of (R, S)-1-phenyl-1,2-ethanediol under non-growing conditions: increase of NADPH availability. J Microbiol Biotechnol 19:65–71. https://doi.org/10.4014/jmb.0804.283
Xu MQ, Wang SS, Li LN, Gao J, Zhang YW, Xu MQ, Wang SS, Li LN, Gao J, Zhang YW (2018) Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 8:460. https://doi.org/10.3390/catal8100460
Kalia VC, Lal S, Rashmi, Chauhan A, Bhattacharyya G (2015) In Silico reconstitution of novel routes for microbial plastic. In: Kalia VC (ed) Microbial factories: biodiversity, biopolymers, bioactive molecules, vol 2. Springer, New Delhi, pp 299–315. https://doi.org/10.1007/978-81-322-2595-9_19
Zhuang MY, Jiang XP, Ling XM, Xu MQ, Zhu YH, Zhang YW (2018) Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J Chem Technol Biotechnol 93:2351–2358. https://doi.org/10.1002/jctb.5579
Jiang XP, Lu TT, Liu CH, Ling XM, Zhuang MY, Zhang JX, Zhang YW (2016) Immobilization of dehydrogenase onto epoxy-functionalized nanoparticles for synthesis of (R)-mandelic acid. Int J Biol Macromol 88:9–17. https://doi.org/10.1016/j.ijbiomac.2016.03.031
Li FL, Shi Y, Zhang JX, Gao J, Zhang YW (2018) Cloning, expression, characterization and homology modeling of a novel water-forming NADH oxidase from Streptococcus mutans ATCC 25175. Int J Biol Macromol 113:1073–1079. https://doi.org/10.1016/j.ijbiomac.2018.03.016
Kim TS, Patel SKS, Selvaraj C, Jung WS, Pan CH, Kang YC, Lee JK (2016) A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep 6:33438. https://doi.org/10.1038/srep33438
Thoden JB, Wohlers TM, Fridovich-Keil JL, Holden HM (2000) Crystallographic evidence for Tyr 157 functioning as the active site base in human UDP-galactose 4-epimerase. Biochemistry 39:5691–5701. https://doi.org/10.1021/bi000215l
Ray S, Kalia VC (2017) Biological significance of degradation of polyhydroxyalkanoates. In: Kalia VC, Kumar P (eds) Microbial applications Vol.1: bioremediation and bioenergy. Springer, Cham, pp 125–139. https://doi.org/10.1007/978-3-319-52666-9_5
Ray S, Sharma R, Kalia VC (2018) Co-utilization of crude glycerol and biowastes for producing polyhydroxyalkanoates. Indian J Microbiol 58:33–38. https://doi.org/10.1007/s12088-017-0702-0
Acknowledgements
This work was supported by Brain Pool Grant (NRF-2018H1D3A2001746) by National Research Foundation of Korea (NRF) to work at Konkuk University. This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2018.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, YH., Liu, CY., Cai, S. et al. Cloning, Expression and Characterization of a Highly Active Alcohol Dehydrogenase for Production of Ethyl (S)-4-Chloro-3-Hydroxybutyrate. Indian J Microbiol 59, 225–233 (2019). https://doi.org/10.1007/s12088-019-00795-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-019-00795-0