Abstract
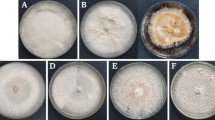
Fungi associated with black point were isolated from three highly susceptible wheat genotypes in the North China Plain. The 21 isolates represented 11 fungal genera. The most prevalent genera were Alternaria (isolation frequency of 56.7%), Bipolaris (16.1%), and Fusarium (6.0%). The other eight genera were Curvularia, Aspergillus, Cladosporium, Exserohilum, Epicoccum, Nigrospora, Penicillium, and Ulocladium; their isolation frequencies ranged from 0.8 to 4.8%. The pathogenicity of the isolates was individually assessed in the greenhouse by inoculating wheat plants with spore suspensions. Ten of the 21 isolates caused significantly higher incidences of black point than that the controls. These isolates belonged to eight fungal species (A. alternata, B. sorokiniana, B. crotonis, B. cynodontis, C. spicifera, F. equiseti, E. rostratum, and E. sorghinum) based on morphological traits and phylogenetic analysis. The average incidences of black point in the eight fungal species were 32.4, 54.3, 43.0, 41.9, 37.2, 38.8, 50.1, and 34.1%, respectively. B. sorokiniana and A. alternata were determined to be the most important pathogens in the North China Plain based on fungal prevalence and symptom severity. This study is the first to identify E. rostratum as a major pathogen causing black point in wheat.


Similar content being viewed by others
References
Yang H, Zehnder A (2001) China’s regional water scarcity and implications for grain supply and trade. Environ Plan A 33:79–95
Conner RL, Thomas JB (1985) Genetic variation and screening techniques for resistance to black point in soft white spring wheat. Can J Plant Pathol 74:402–407
Sissons M, Sissons S, Egan N (2010) The black point status of selected tetraploid species and Australian durum wheat and breeding lines. Crop Sci 50:1279–1286
Rees RG, Martin DJ, Law DP (1984) Black point in bread wheat: effects on quality and germination and fungal associations. Aust J Exp Agric 127:601–605
Conner RL, Hwang SF, Stevens RR (1996) Fusarium proliferatum: a new causal agent of black point in wheat. Can J Plant Pathol 18:419–423
Li QY, Qin Z, Jiang YM, Shen CC, Duan ZB, Niu JS (2014) Screening wheat genotypes for resistance to black point and the effects of diseased kernels on seed germination. J Plant Dis Prot 121:79–88
El-Gremi SM, Draz IS, Youssef WAE (2017) Biological control of pathogens associated with kernel black point disease of wheat. Crop Prot 91:13–19
Sisterna MN, Sarandon SJ (2005) Preliminary studies on the natural incidence of wheat black point under different fertilization levels and tillage systems in Argentina. Plant Pathol J 4:26–28
Malaker PK, Mian IH (2010) Population dynamics of mycoflora and incidence of black point disease in wheat grains. Bangladesh J Agric Res 35:1–10
Zadoks J, Chang T, Konzak C (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Lu JY (2001) Plant pathogenic mycology. China Agriculture Press, Beijing (in Chinese)
Manamgoda DS, Rossman AY, Castlebury LA, Crous PW, Madrid H, Chukeatirote E, Hyde KD (2014) The genus Bipolaris. Stud Mycol 79:221–288
Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, Jones EB, Kodsueb R, Phookamsak R, Chukeatirote E, Bahkali AH, Hyde KD (2011) Capnodiaceae. Fungal Divers 51:103–134
Mahto BN, Gurung S, Adhikari TB (2011) Assessing genetic resistance to spot blotch, stagonospora nodorum blotch and tan spot in wheat from Nepal. Eur J Plant Pathol 131:249–260
Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD (2012) A phylogenetic and taxonomic re-evaluation of the Bipolaris–Cochliobolus–Curvularia complex. Fungal Divers 56:131–144
Woudenberg JHC, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212
Lee YM, Hong JH, Lee H, Ahn BJ, Kim GH, Kim JJ (2013) Phylogenetic analysis of the genus Fusarium and their antifungal activity against wood-decay and sapstain fungi. Holzforschung 67:473–478
Chowdhary A, Hagen F, Curfsbreuker I, Madrid H, Hoog GSD, Meis JF (2015) In vitro activities of eight antifungal drugs against a global collection of genotyped Exserohilum isolates. Antimicrob Agents Chemother 59:6642–6645
Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW (2015) Resolving the Phoma enigma. Stud Mycol 82:137–217
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Nylander JAA (2004) Mr Model Test v2. Evolutionary Biology Center, University of Uppsala, Uppsala
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Huelsenbeck JP, Ronquist F (2005) Bayesian analysis of molecular evolution using MrBayes. In: Nielsen R (ed) Statistical methods in molecular evolution. Springer, New York, pp 183–232
Williamson PM (1997) Black point of wheat. In vitro production of symptoms, enzymes involved, and association with Alternaria alternata. Aust J Agric Res 48:13–19
Clarke MP, Gooding MJ, Jones SA (2004) The effects of irrigation, nitrogen fertilizer and grain size on Hagberg falling number, specific weight and black point of winter wheat. J Sci Food Agric 84:227–236
Fernandez MR, Sissons M, Conner RL, Wang H, Clarke JM (2011) Influence of biotic and abiotic factors on dark discoloration of durum wheat kernels. Crop Sci 51:1205–1214
Acknowledgments
This study was funded by the National Science and Technology Pillar Program during the 12th five-year plan period (Grant Number 2015BAD26B01) and the scientific and technological program of Henan province in China (Grant Number 172102110041).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, KG., Jiang, YM., Li, YK. et al. Identification and Pathogenicity of Fungal Pathogens Causing Black Point in Wheat on the North China Plain. Indian J Microbiol 58, 159–164 (2018). https://doi.org/10.1007/s12088-018-0709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-018-0709-1