Abstract
Introduction
Humans are very sensitive to several bitter compounds, although there is great inter-individual variability in the elicited emotional (e.g., disgust) and somatic (e.g., nausea) responses. The variability might be associated with the personality trait “disgust proneness” (DP; general tendency to respond with the emotion of disgust).
Method
This study examined the relationship between the sensitivity to quinine hydrochloride and DP in children (63 boys and 67 girls; mean age = 8.5 years) and adults (107 men, 95 women; mean age = 29.4 years). Each participant rated the perceived taste intensity of four gustatory stimuli (sucrose, 0.4 g/ml; sodium chloride, 0.25 g/ml; quinine hydrochloride, 0.006 g/ml; citric acid, 0.3 g/ml) and completed questionnaires that assessed the proneness to experience disgust, anxiety, and depressed mood.
Results
The computed multiple linear regression analyses for the adults showed that DP but no other trait measure was associated with bitter sensitivity. In children, depression proneness and age were predictors of bitter sensitivity.
Conclusions
This study revealed age-dependent correlations between DP and bitter sensitivity.
Implications
This finding points to the dynamic features of taste sensitivity (and possibly DP) across the lifespan. Therefore, longitudinal studies are warranted.
Similar content being viewed by others
Introduction
Bitter taste perception plays an important role in food preferences and dietary habits (e.g., Tepper et al. 2017). Especially bitterness of high intensity is often considered disgusting and leads to food rejection (Rozin et al. 2009). From an evolutionary perspective, it has been argued that bitter-tasting substances elicit innately determined oral rejection responses because bitterness signals potential health threat (e.g., Rosenstein and Oster 1988). Spoiled or toxic food often tastes bitter and therefore needs to be spat out. In line with this conception is a study by Peyrot et al. (2011). The participants were presented with four concentrated solutions (bitter, salty, sweet, unami) that were held in the mouth for 3 min each and then expectorated. Only the bitter taste induced nausea showing that the body not only detects potential toxins but anticipates their ingestion by eliciting a prophylactic aversive state.
Despite the function of a bitter taste as a warning signal of possible food toxicity, bitter responses in humans (i.e., the perceived intensity of a bitter compound) show pronounced inter-individual variability. This variation is associated with different factors, such as the genotype of the bitter taste receptors (TAS2Rs), sex, age, and personality traits that are associated with affective processing (e.g., Bartoshuk et al. 1994; Goldstein et al. 2007; Herbert et al. 2014). In humans, one of the best-known examples of marked differences in bitter perception is the ability to taste 6-n-propylthiouracil (PROP). Typically, three sensitivity groups are distinguished from each other: PROP nontasters, tasters, and supertasters (e.g., Bartoshuk et al. 1994). Several studies observed differences in PROP detection related to sex and age (for a review see Tepper et al. 2017). Women are more likely to be supertasters than men, and older individuals are usually less bitter-sensitive than younger ones (e.g., Bartoshuk et al. 1994; Goldstein et al. 2007). These differences are however small. In general, taste does appear to be relatively stable across the lifespan. Moreover, the slight age-related decline in bitter sensitivity seems to be compound-specific. In a study by Cowart et al. (1994), older adults (relative to younger adults) showed a reduced sensitivity for quinine but not for urea. A study by Mennella et al. (2005) revealed that the genotype-phenotype-relationship concerning PROP sensitivity was modified by age. Among TAS2R38 heterozygotes, children were more responsive to PROP than adults. These data suggest that the penetrance of this gene varies with age. Individuals who are born sensitive to PROP may become less sensitive over time because of habituation and aging effects.
Finally, previous research indicated that PROP sensitivity is associated with the personality trait “disgust proneness” (DP; general tendency of a person to experience disgust across different situations; Schienle et al. 2002). For example, Herz (2011) demonstrated that PROP supertasters were more prone to visceral disgust than PROP nontasters. Similarly, in a study by Herbert et al. (2014), PROP tasters reported a higher DP toward body products than nontasters. Different authors have argued that disgust evolved from the bitter taste rejection response (e.g., Rozin et al. 2009). Therefore, a correlation between DP and bitter sensitivity seems logical. Such a correlation should be present not only in adults but also in children because the bitter rejection response is already present in newborns (e.g., Rosenstein and Oster 1988). However, children are not merely miniature adults. Their chemosensory systems mature postnatally and undergo changes associated with learning and experience. Therefore, it still needs to be demonstrated if a positive correlation between DP and bitter sensitivity is also present in children.
Recently, it has been questioned whether bitter sensitivity can be considered a homogenous trait. For example, Roura et al. (2015) administered eight different bitter compounds to their subjects (PROP, sinigrin, saccharin, caffeine, quassia extract, gentian extract, and quinine hydrochloride). They found that PROP sensitivity was not a reliable predictor of general bitter sensitivity. In addition, PROP sensitivity seems to be associated with generally heightened taste sensations. Hayes et al. (2008) reported that PROP-sensitive participants perceived quinine as more intense but also sucrose, citric acid, and sodium chloride (even after statistically controlling for TAS2R38 genotype).
The aim of the present study was to investigate whether quinine sensitivity is associated with DP. Quinine is an alkaloid derived from the bark of the cinchona tree. Its bitterness value is 200,000 (Eisenbrand and Schreier 2006). Due to its low water solubility, it is usually administered as a salt (quinine HCl) in standardized gustatory tests (e.g., Landis et al. 2009). Humans are very sensitive to quinine HCl with a detection threshold in the micromolar range (Peyrot et al. 2011).
In the present study, a multiple linear regression approach was chosen in order to capture the relationship between bitter sensitivity to quinine HCl and DP in children and adults. We also assessed the sensitivity to sweet, sour, and salty taste, and the personality characteristics depression/ anxiety proneness in order to investigate the taste/emotion specificity of the observed associations. In previous studies, the perceived intensity of quinine showed small positive correlations with citric acid, sucrose, and NaCl (e.g., Lim et al. 2008). Moreover, DP is moderately correlated with habitual affective negativity, including trait anxiety and emotional lability/neuroticism (for a summary, see, Schienle et al. 2002). Therefore, these variables were also introduced as predictors in the regression analyses.
Method
Samples
Adult sample
A total of 202 subjects (95 women, 107 men) with a mean age of M = 29.42 years (SD = 11.37) participated in the study. The majority had a high school diploma (87 %) and were white-collar workers; the others were blue-collar workers. All participants gave written informed consent.
Children sample
A total of 130 children (63 boys and 67 girls) with a mean age of 8.5 years (SD = 1.08; range, 7–11 years) participated in the study. The children were recruited from elementary schools and afterschool day care programs. Informed consent was provided by the teachers, the parents, and the children.
For both samples, reported chronic diseases (e.g., asthma) and acute nasal diseases (e.g., acute infections) led to exclusion from the investigation.
Gustatory Test
All participants were tested individually with a standardized gustatory test (according to Landis et al. 2009). The test included four stimuli that were presented with the following concentrations: sucrose, 0.4 g/ml; sodium chloride, 0.25 g/ml; quinine hydrochloride, 0.006 g/ml; and citric acid, 0.3 g/ml. Distilled water was used as the solvent. Prior to administering a gustatory stimulus, the mouth was rinsed with water. The participants were asked to label each taste based on a list with four descriptors, i.e., sweet, sour, salty, and bitter (multiple forced choice). In addition, the participants used the general Labeled Magnitude Scale (gLMS; Bartoshuk et al. 2003) to report the intensity of the fluids. The gLMS ranges from no sensation (0) to “the strongest imaginable sensation of any kind” (100).
Two hours prior to testing, the participants were asked not to eat or drink (except water).
Questionnaires
Adults
The participants completed the Questionnaire for the Assessment of Disgust Proneness (QADP; Schienle et al. 2002) and the subscales Depression and Anxiety of the Brief Symptom Inventory (BSI-18; German version by Spitzer et al. 2011) via an online survey. The QADP describes 37 situations (e.g., “someone with bad breath speaks to you”), which have to be judged on 5-point scales regarding experienced disgust (0 = “not disgusting”; 4 = “very disgusting”). The QADP had a Cronbach’s alpha of .94 in the present sample. The BSI-18 is a self-report instrument for psychological problems and their intensity during the last week. The subscales Depression (α = .85) and Anxiety (α = .73) have six items each that are scored on a five-point Likert scale ranging from 0 (not at all) to very strong (4).
Children
The children answered German versions of the subscale Core Disgust of the Questionnaire for the Assessment of Disgust Proneness for Children (QADP-C; Schienle and Rohrmann 2012) and the Depression Inventory for Children and Adolescents (DICA; Stiensmeier-Pelster et al. 2000). The subscale Core Disgust of the QADP-C assesses experienced disgust to spoiled food and poor hygiene. The eight items (e.g., “someone with bad breath speaks to you”; α = .86) are rated on 5-point scales (0 = “not disgusting” to 4 = “very disgusting”). The DICA is a 26-item screening instrument for depressive symptoms (α = .84). The items are rated via a three-alternative forced choice format (e.g., “most of what I do works out/ a lot of what I do is wrong/ everything I do is wrong”).
A subsample of 77 children (38 boys and 39 girls with a mean age of 8.2 years; SD = 1.06) additionally completed the Screen for Child Anxiety and Related Emotional Disorders (SCARED; Essau et al. 2002). The SCARED has 41 items (e.g., “when I feel frightened, my breathing becomes difficult”) that are rated on 3-point scales (0 = “not true or hardly ever true”; 2 = “very true or often true”). The reliability (Cronbach’s alpha) of the total scale was α = .87 in the present sample.
The study with both samples (adults, children) had been approved by the local ethical committee and was performed according to the guidelines of the Declaration of Helsinki.
Statistical Analyses
For the sample of adults, four multiple linear regression analyses were calculated to capture the association between the perceived intensity of the four basic tastes (salty, sour, sweet, and bitter) and the predictors’ disgust proneness, depression proneness, anxiety proneness, sex, and age. The “enter method” was chosen; all independent variables were entered into the equation at the same time (forced entry).
For the children, four multiple linear regression analyses (forced entry) were computed with the same predictors (except for anxiety proneness because only a subsample had answered the SCARED). The models were assessed for multicollinearity and residual distribution. The analyses were conducted with SPSS version 24 (IBM Corp. 2016).
Results
Taste Labeling
Adults
Percentages of correct classifications were as follows: salty (98 %), sweet (98 %), sour (97 %), and bitter (99 %). The percentages did not differ between the four taste categories (Cochran’s χ2(3) = 2.40, p = .494).
Children
The correct classifications did not differ between the four taste categories (χ2(3) = 3.21, p = .361): salty (85 %), sweet (82 %), sour (79 %), and bitter (78 %).
Adults showed a better classification performance than children for all basic tastes (salty taste, χ2(1) = 21.06; p < .001; sour taste, χ2(1) = 29.67, p < .001; sweet taste, χ2(1) = 26.14, p < .001; bitter taste, χ2(1) = 42.46, p < .001).
Taste Intensity
Descriptive statistics (means, standard deviations) for the intensity ratings (%) are displayed in Table 1 for both children and adults.
Adults
The ranges of intensity ratings for the four basic tastes were as follows: quinine HCl (1–100 %), NaCl (10–100 %), sucrose (10–100 %), and citric acid (20–100 %). Women rated the intensity of salt and quinine HCl higher than men. There were no sex differences for citric acid and sucrose (see, Table 1).
Children
The ranges of intensity ratings for the four basic tastes were as follows: quinine HCl (2–100 %), NaCl (1–100 %), sucrose (10–100 %), citric acid (2–100 %). Boys rated the intensity of sucrose higher than girls. There were no sex differences for citric acid, NaCl, and quinine HCl (see, Table 1).
Independent sample t tests showed that children rated sucrose as more intense than adults (p = .04). Regarding the other stimuli (NaCl, citric acid, quinine HCl), children and adults did not differ in their intensity ratings (all p > .27).
The perceived intensity of quinine HCl showed small to moderate correlations with the other basic tastes in children as well as adults (r = .21–.49). The correlation coefficients are displayed in the supplementary Table 1.
Questionnaires
Descriptive statistics (means, standard deviations) for the questionnaires are displayed in Table 1 for children and adults.
Adults
Relative to men, women scored higher on the QADP and BSI_Anxiety but did not differ in self-reports for depression proneness (BSI_Depression). The obtained mean scores of the questionnaires were not elevated compared to the scores of normative (construction) samples (all p > .05).
The computed Pearson correlations between the questionnaire scores were as follows: QADP – BSI_Anxiety: r = .20 (p = .005); QADP – BSI_Depression: r = .17 (p = .017); BSI_Anxiety – BSI_Depression: r = .67 (p < .001).
Children
Boys scored higher on the DICA and lower on the QADP-C than girls. SCARED scores did not differ between boys and girls. The present sample obtained lower scores on the DICA (p = .04), higher QADP-C scores (p = .001), as well as higher SCARED scores (p < .001) than the normative (construction) samples.
The computed Pearson correlations between the questionnaire scores were as follows: QADP-C – SCARED: r = .21 (p = .07); QADP-C – DIKJ: r = −.01 (.96); SCARED – DIKJ: r = .35 (p = .002).
Multiple Linear Regression Analyses
Adults
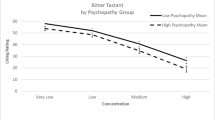
For the criterion bitter sensitivity (perceived intensity of quinine HCl), a significant regression equation was found (F(5,196) = 3.70; p = .003; R2 = .09). Disgust proneness was a positive predictor of bitter sensitivity (see, Table 2), whereas the other variables (age, sex, symptoms of anxiety, symptoms of depression) were not relevant (see, Table 2).
For salt sensitivity, a significant regression equation was found (F(5,195) = 3.00; p = .02; R2 = .07). Sex was the only significant predictor with women reporting a higher intensity (B = − .972, SE B = 4.38, p = .024; all other predictors p > .05). The regression equations for sweet/sour sensitivity were not significant (ps > .05).
Children
A significant regression equation was found for the criterion “perceived intensity of quinine HCl” (F(4,129) = 4.25; p = .003; R2 = .12). Age was a positive predictor of bitter sensitivity and depression proneness (DICA) a negative predictor, whereas disgust proneness and sex were not relevant (see, Table 2). This finding was still present after exclusion of children with incorrect bitter labeling (F(4,100) = 3.18, p = .017; R2 = .12).
For sweet sensitivity, a significant regression equation was found (F(4,129) = 2.44; p = .05; R2 = .07). Age was the only significant predictor with older children reporting higher intensity (B = 5.23, SE B = 2.60, p = .046). The regression equations for sensitivity for sour and salty taste were not significant (ps > .05).
In order to investigate the association between anxiety proneness (SCARED) and taste sensitivity, we computed correlations for the subsample of 77 children. The correlation coefficients were as follows (salty: r = − .070, p = .565; sour: r = − .150, p = .182; sweet: r = − .230, p = .042; bitter: r = − .09; p = .449).
Discussion
This investigation focused on the association between bitter sensitivity (perceived intensity of quinine HCl) and disgust proneness in children and adults.
The computed regression analyses with the data from adults showed that only disgust proneness (QADP score) correlated with bitter sensitivity but none of the other affective personality variables (proneness to experience anxiety, depressed mood). The QADP mainly contains items related to possible pathogen transmission. The total score reflects fear of contamination. Some researchers (e.g., Schaller and Duncan 2007) have suggested that disgust is a central element of the “behavioral immune system,” which allows individuals to detect the potential presence of disease-causing agents and to engage in behaviors that prevent contact with contaminated (food) items. Consequently, persons high in DP should have a greater sensitivity to detect the risk of foodborne illness (as indexed by bitter taste). The present study extends previous research (e.g., Herz 2011; Herbert et al. 2014) and demonstrates that not only PROP sensitivity but also quinine HCl sensitivity is related to disgust propensity in adults.
In children, depression proneness and age were associated with bitter sensitivity but not DP. Older children and children with lower depression scores rated quinine HCl as more intense. These results clearly differ from the findings for the adult sample. Different factors might be relevant within this context.
First, it has to be noted that the gustatory classification performance was better in adults as compared to children with the greatest difference regarding the bitter taste (children, 78 % vs. adults, 99 % correct labels). This finding may be related to the gustatory experience. For example, beverages that contain quinine HCl (e.g., tonic water, bitter lemon) are predominantly consumed by adults. Despite this difference in correct labeling between children and adults, both groups gave comparable intensity ratings for quinine HCl. In addition, the results of the regression analysis for the children did not change after exclusion of participants with incorrect bitter labels.
Second, the studied children sample reported below-average depression proneness (DICA score). Nevertheless, the DICA was a negative predictor of bitter sensitivity. Children, who reported less negative feelings and symptoms (e.g., loneliness, reduced appetite, sleep problems, feelings of incompetence at school) were characterized by higher sensitivity to quinine. This finding may point to the relevance of general somatic and affective well-being for bitter detection in childhood. Future studies should include pediatric samples in order to elucidate the relationship between clinically relevant symptoms of depression and bitter perception.
Third, the children reported above-average disgust proneness (QADP-C) as compared to the construction sample (Schienle and Rohrmann 2012). The QADP-C had been developed based on the items of the questionnaire for adults (QADP; Schienle et al. 2002) with child-adapted formulations. Thus, in the present investigation, corresponding disgust constructs were assessed in children and adults. This raises the question of why DP and bitter sensitivity were correlated in adults but not in children. It is possible that environmental factors are more important in childhood than later in life. For another chemosensory system (olfaction), Nováková et al. (2018) showed that the diversity of children’s everyday olfactory environment may affect the development of their olfactory abilities and odor awareness. Very similar, children need to experience different tastes and flavors in order to be able to develop likes and dislikes for certain food items. It is therefore not surprising that the PROP taster status is not as closely linked to (bitter) food choices in children compared to adults (e.g., Mennella et al. 2005; O'Brien et al. 2013; Tepper et al. 2017).
Altogether, these findings point to the dynamic features of taste sensitivity (and possibly DP) across the lifespan. Especially for children samples, longitudinal studies are warranted. We investigated children aged 7 to 11 years. Correlations between DP and bitter sensitivity might change over time. This has to be addressed in a future study.
References
Bartoshuk LM, Duffy VB, Miller IJ (1994) PTC/PROP tasting: anatomy psychophysics and sex effects. Physiol Behav 56:1165–1171. https://doi.org/10.1016/0031-9384(94)90361-1
Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ (2003) Labeled scales (eg, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Prefer 14:125–138
Cowart BJ, Yokomukai Y, Beauchamp GK (1994) Bitter taste in aging: compound-specific decline in sensitivity. Physiol Behav 56:1237–1241
Eisenbrand G, Schreier P (2006) RÖMPP Lexikon Lebensmittelchemie. Thieme, Stuttgart. https://doi.org/10.1002/food.19950390311
Essau CA, Muris P, Ederer EM (2002) Reliability and validity of the Spence Children’s Anxiety Scale and the Screen for Child Anxiety Related emotional disorders in German children. J Behav Ther Exp Psychiatry 33:1–18. https://doi.org/10.1016/S0005-7916(02)00005-8
Goldstein GL, Daun H, Tepper BJ (2007) Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol Behav 90:809–817. https://doi.org/10.1016/j.physbeh.2007.01.004
Hayes JE, Batoshuk LM, Kidd RK, Duffy VB (2008) Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses 33:255–265
Herbert C, Platte P, Wiemer J, Macht M, Blumenthal TD (2014) Supertaster super reactive: oral sensitivity for bitter taste modulates emotional approach and avoidance behavior in the affective startle paradigm. Physiol Behav 135:198–207. https://doi.org/10.1016/j.physbeh.2014.06.002
Herz RS (2011) PROP taste sensitivity is related to visceral but not moral disgust. Chemosens Percept 4:72–79. https://doi.org/10.1007/s12078-011-9089-1
IBM Corp. Released (2016) IBM SPSS Statistics for Windows. Version 24. Armonk, NY. IBM Corp
Landis BN, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T (2009) “Taste Strips”–a rapid lateralized gustatory bedside identification test based on impregnated filter papers. J Neurol 256:242. https://doi.org/10.1007/s00415-009-0088-y
Lim JY, Urban L, Green BG (2008) Measures of individual differences in taste and creaminess perception. Chem Senses 33:493–501
Mennella JA, Pepino MY, Reed DR (2005) Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115:216–222. https://doi.org/10.1542/peds.2004-1582
Nováková LM, Fialová J, Havlíček J (2018) Effects of diversity in olfactory environment on children’s sense of smell. Sci Rep 8:2937. https://doi.org/10.1038/s41598-018-20236-0
O'Brien SA, Feeney EL, Scannell AG, Markey A, Gibney ER (2013) Bitter taste perception and dietary intake patterns in Irish children. J Nutrigenet Nutrigenomics 6:43–58. https://doi.org/10.1159/000348442
Peyrot d, Gachons C, Beauchamp GK, Stern RM, Koch KL, Breslin PAS (2011) Bitter taste induces nausea. Curr Biol 21:247–248
Rosenstein D, Oster H (1988) Differential facial responses to four basic tastes in newborns. Child Dev:1555–1568. https://doi.org/10.2307/1130670
Roura E, Aldayyani A, Thavaraj P, Prakash S, Greenway D, Thomas WG, Meyerhof W, Roudnitzky FSR (2015) Variability in human bitter taste sensitivity to chemically diverse compounds can be accounted for by differential TAS2R activation. Chem Senses 40:427–435. https://doi.org/10.1093/chemse/bjv024
Rozin P, Haidt J, Fincher K (2009) From oral to moral. Science 323:1179–1180. https://doi.org/10.1126/science.1170492
Schaller M, Duncan LA (2007) The behavioral immune system: its evolution and social psychological implications. In: Forgas JP, Haselton MG, von Hippel W (eds) Evolution and the social mind: Evolutionary psychology and social cognition. Psychology Press, New York, pp 293–307. https://doi.org/10.4324/9780203837788
Schienle A, Rohrmann S (2012) Ein Fragebogen zur Erfassung der Ekelempfindlichkeit bei Kindern (FEEK). Klin Diagn Eval 4:379–392
Schienle A, Walter B, Stark R, Vaitl D (2002) Ein Fragebogen zur Erfassung der Ekelempfindlichkeit (FEE). Z Klin Psychol Psychother 31:110–120. https://doi.org/10.1026//1616-3443.31.2.110
Spitzer C, Hammer S, Löwe B, Grabe HJ, Barnow S, Rose M, Wingenfeld K, Freyberger HJ, Franke GH (2011) The short version of the Brief Symptom Inventory (BSI-18): preliminary psychometric properties of the German translation. Fortschr Neurol Psychiatr 79:517–523. https://doi.org/10.1055/s-0031-1281602
Stiensmeier-Pelster J, Schürmann M, Duda K (2000) DIKJ: Depressionsinventar für Kinder und Jugendliche. 2. Aufl. Hogrefe, Göttingen
Tepper BJ, Melis M, Koelliker Y, Gasparini P, Ahijevych KL, Barbarossa IT (2017) Factors influencing the phenotypic characterization of the oral marker PROP. Nutrients 9:1–15. https://doi.org/10.3390/nu9121275
Funding
Open access funding provided by University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All participants (and parents of the children) gave written informed consent. The study had been approved by the ethics committee of the University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schienle, A., Schlintl, C. The Association Between Quinine Hydrochloride Sensitivity and Disgust Proneness in Children and Adults. Chem. Percept. 13, 78–83 (2020). https://doi.org/10.1007/s12078-019-09268-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-019-09268-6