Abstract
Background
Lowered nicotinamide adenine dinucleotide (NAD+) levels in tumor cells drive tumor hyperprogression during immunotherapy, and its restoration activates immune cells. However, the effect of lenvatinib, a first-line treatment for unresectable hepatocellular carcinoma (HCC), on NAD+ metabolism in HCC cells, and the metabolite crosstalk between HCC and immune cells after targeting NAD+ metabolism of HCC cells remain unelucidated.
Methods
Liquid chromatography-tandem mass spectrometry (LC–MS/MS) and ultra-high-performance liquid chromatography multiple reaction monitoring-mass spectrometry (UHPLC-MRM-MS) were used to detect and validate differential metabolites. RNA sequencing was used to explore mRNA expression in macrophages and HCC cells. HCC mouse models were used to validate the effects of lenvatinib on immune cells and NAD+ metabolism. The macrophage properties were elucidated using cell proliferation, apoptosis, and co-culture assays. In silico structural analysis and interaction assays were used to determine whether lenvatinib targets tet methylcytosine dioxygenase 2 (TET2). Flow cytometry was performed to assess changes in immune cells.
Results
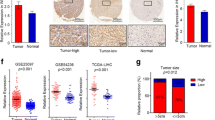
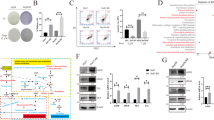
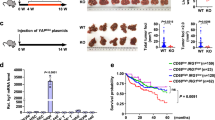
Lenvatinib targeted TET2 to synthesize and increase NAD+ levels, thereby inhibiting decomposition in HCC cells. NAD+ salvage increased lenvatinib-induced apoptosis of HCC cells. Lenvatinib also induced CD8+ T cells and M1 macrophages infiltration in vivo. And lenvatinib suppressed niacinamide, 5-Hydroxy-L-tryptophan and quinoline secretion of HCC cells, and increased hypoxanthine secretion, which contributed to proliferation, migration and polarization function of macrophages. Consequently, lenvatinib targeted NAD+ metabolism and elevated HCC-derived hypoxanthine to enhance the macrophages polarization from M2 to M1. Glycosaminoglycan binding disorder and positive regulation of cytosolic calcium ion concentration were characteristic features of the reverse polarization.
Conclusions
Targeting HCC cells NAD+ metabolism by lenvatinib-TET2 pathway drives metabolite crosstalk, leading to M2 macrophages reverse polarization, thereby suppressing HCC progression. Collectively, these novel insights highlight the role of lenvatinib or its combination therapies as promising therapeutic alternatives for HCC patients with low NAD+ levels or high TET2 levels.






Similar content being viewed by others
Data availability
The RNA sequencing data and non-target metabolomics data can be shared if anyone interests and provides Email address.
References
Chen D, Liu J, Zang L, Xiao T, Zhang X, Li Z, et al. Integrated machine learning and bioinformatic analyses constructed a novel stemness-related classifier to predict prognosis and immunotherapy responses for hepatocellular carcinoma patients. Int J Biol Sci. 2022;18(1):360–373. https://doi.org/10.7150/ijbs.66913
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654
Vogl TJ, Zangos S, Balzer JO, Nabil M, Rao P, Eichler K, et al. transarterial chemoembolization (tace) in hepatocellular carcinoma: technique, indication and results. Rofo. 2007;179(11):1113–1126. https://doi.org/10.1055/s-2007-963285
Chen S, Zeng X, Su T, Xiao H, Lin M, Peng Z, et al. Combinatory local ablation and immunotherapies for hepatocellular carcinoma: rationale, efficacy, and perspective. Front Immunol. 2022;13:1033000. https://doi.org/10.3389/fimmu.2022.1033000
D’Alessio A, Cammarota A, Prete MG, Pressiani T, Rimassa L. The evolving treatment paradigm of advanced hepatocellular carcinoma: putting all the pieces back together. Curr Opin Oncol. 2021;33(4):386–394. https://doi.org/10.1097/CCO.0000000000000744
Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in keynote-240: a randomized, double-blind, phase iii trial. J Clin Oncol. 2020;38(3):193–202. https://doi.org/10.1200/JCO.19.01307
Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022. https://doi.org/10.1038/s41575-022-00704-9
Chen R, Li Q, Xu S, Ye C, Tian T, Jiang Q, et al. Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: from modulation to combination therapy targeting the microenvironment. Cancer Cell Int. 2022;22(1):73. https://doi.org/10.1186/s12935-021-02435-4
Tummala KS, Gomes AL, Yilmaz M, Graña O, Bakiri L, Ruppen I, et al. Inhibition of de novo nad(+) synthesis by oncogenic uri causes liver tumorigenesis through dna damage. Cancer Cell. 2014;26(6):826–839. https://doi.org/10.1016/j.ccell.2014.10.002
Li G, Choi JE, Kryczek I, Sun Y, Liao P, Li S, et al. Intersection of immune and oncometabolic pathways drives cancer hyperprogression during immunotherapy. Cancer Cell. 2023. https://doi.org/10.1016/j.ccell.2022.12.008
Lv H, Lv G, Chen C, Zong Q, Jiang G, Ye D, et al. Nad(+) metabolism maintains inducible pd-l1 expression to drive tumor immune evasion. Cell Metab. 2021;33(1):110-127.e5. https://doi.org/10.1016/j.cmet.2020.10.021
Wang Y, Wang F, Wang L, Qiu S, Yao Y, Yan C, et al. Nad(+) supplement potentiates tumor-killing function by rescuing defective tub-mediated nampt transcription in tumor-infiltrated t cells. Cell Rep. 2021;36(6):109516. https://doi.org/10.1016/j.celrep.2021.109516
Guo X, Tan S, Wang T, Sun R, Li S, Tian P, et al. Nad(+) salvage governs mitochondrial metabolism, invigorating natural killer cell antitumor immunity. Hepatology. 2022. https://doi.org/10.1002/hep.32658
Xiao H, Guo Y, Li B, Li X, Wang Y, Han S, et al. M2-like tumor-associated macrophage-targeted codelivery of stat6 inhibitor and ikkβ sirna induces m2-to-m1 repolarization for cancer immunotherapy with low immune side effects. ACS Cent Sci. 2020;6(7):1208–1222. https://doi.org/10.1021/acscentsci.9b01235
Wang Y, Tiruthani K, Li S, Hu M, Zhong G, Tang Y, et al. Mrna delivery of a bispecific single-domain antibody to polarize tumor-associated macrophages and synergize immunotherapy against liver malignancies. Adv Mater 2021;33(23):e2007603. https://doi.org/10.1002/adma.202007603.
Denardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. https://doi.org/10.1038/s41577-019-0127-6
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. https://doi.org/10.1016/j.coi.2010.01.009
Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. https://doi.org/10.1016/j.it.2011.12.001
Liu N, Wang X, Steer CJ, Song G. Microrna-206 promotes the recruitment of cd8(+) t cells by driving m1 polarisation of kupffer cells. Gut. 2022;71(8):1642–1655. https://doi.org/10.1136/gutjnl-2021-324170
Wei CY, Zhu MX, Zhang PF, Huang XY, Wan JK, Yao XZ, et al. Pkcα/zfp64/csf1 axis resets the tumor microenvironment and fuels anti-pd1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77(1):163–176. https://doi.org/10.1016/j.jhep.2022.02.019
Katsyuba E, Romani M, Hofer D, Auwerx J. Nad(+) homeostasis in health and disease. Nat Metab. 2020;2(1):9–31. https://doi.org/10.1038/s42255-019-0161-5
Covarrubias AJ, Perrone R, Grozio A, Verdin E. Nad(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–141. https://doi.org/10.1038/s41580-020-00313-x
Ogiya D, Liu J, Ohguchi H, Kurata K, Samur MK, Tai YT, et al. The jak-stat pathway regulates cd38 on myeloma cells in the bone marrow microenvironment: therapeutic implications. Blood. 2020;136(20):2334–2345. https://doi.org/10.1182/blood.2019004332
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, et al. Tet2 is required to resolve inflammation by recruiting hdac2 to specifically repress il-6. Nature. 2015;525(7569):389–393. https://doi.org/10.1038/nature15252
Parsons RB, Kocinaj A, Ruiz PG, Prendergast SA, Parsons AE, Facey PD, et al. Alpha-synucleinopathy reduces nmnat3 protein levels and neurite formation that can be rescued by targeting the nad+ pathway. Hum Mol Genet. 2022;31(17):2918–2933. https://doi.org/10.1093/hmg/ddac077
Chiarugi A, Dölle C, Felici R, Ziegler M. The nad metabolome–a key determinant of cancer cell biology. Nat Rev Cancer. 2012;12(11):741–752. https://doi.org/10.1038/nrc3340
Chua G, Wassarman KL, Sun H, Alp JA, Jarczyk EI, Kuzio NJ, et al. Cytosine-based tet enzyme inhibitors. Acs Med Chem Lett. 2019;10(2):180–185. https://doi.org/10.1021/acsmedchemlett.8b00474
Singh AK, Zhao B, Liu X, Wang X, Li H, Qin H, et al. Selective targeting of tet catalytic domain promotes somatic cell reprogramming. Proc Natl Acad Sci U S A. 2020;117(7):3621–3626. https://doi.org/10.1073/pnas.1910702117
Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo nad(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20(1):50–63. https://doi.org/10.1038/s41590-018-0255-3
Xu YP, Lv L, Liu Y, Smith MD, Li WC, Tan XM, et al. Tumor suppressor tet2 promotes cancer immunity and immunotherapy efficacy. J Clin Invest. 2019;129(10):4316–4331. https://doi.org/10.1172/JCI129317
Li S, Feng J, Wu F, Cai J, Zhang X, Wang H, et al. Tet2 promotes anti-tumor immunity by governing g-mdscs and cd8(+) t-cell numbers. Embo Rep 2020;21(10):e49425. https://doi.org/10.15252/embr.201949425.
Yang G, Zeng X, Wang M, Wu A. The tet2/e-cadherin/β-catenin regulatory loop confers growth and invasion in hepatocellular carcinoma cells. Exp Cell Res. 2018;363(2):218–226. https://doi.org/10.1016/j.yexcr.2018.01.011
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. https://doi.org/10.1016/j.cell.2017.01.017
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217
Kamerkar S, Leng C, Burenkova O, Jang SC, Mccoy C, Zhang K, et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoaso-stat6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8(7):eabj7002. https://doi.org/10.1126/sciadv.abj7002
Ho DW, Tsui YM, Chan LK, Sze KM, Zhang X, Cheu JW, et al. Single-cell rna sequencing shows the immunosuppressive landscape and tumor heterogeneity of hbv-associated hepatocellular carcinoma. Nat Commun. 2021;12(1):3684. https://doi.org/10.1038/s41467-021-24010-1
Hao X, Zheng Z, Liu H, Zhang Y, Kang J, Kong X, et al. Inhibition of apoc1 promotes the transformation of m2 into m1 macrophages via the ferroptosis pathway and enhances anti-pd1 immunotherapy in hepatocellular carcinoma based on single-cell rna sequencing. Redox Biol. 2022;56:102463. https://doi.org/10.1016/j.redox.2022.102463
Chang Z, Zhang Q, Hu Q, Liu Y, Zhang L, Liu R. Tannins in terminalia bellirica inhibits hepatocellular carcinoma growth via re-educating tumor-associated macrophages and restoring cd8(+)t cell function. Biomed Pharmacother. 2022;154:113543. https://doi.org/10.1016/j.biopha.2022.113543
Zhang Y, Vu T, Palmer DC, Kishton RJ, Gong L, Huang J, et al. A t cell resilience model associated with response to immunotherapy in multiple tumor types. Nat Med. 2022;28(7):1421–1431. https://doi.org/10.1038/s41591-022-01799-y
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 82172751), China Postdoctoral Science Foundation (Grant No. 2021M701625), President Foundation of Nanfang Hospital, Southern Medical University (Grant No. 2021C003), and Guangdong Natural Science Foundation (Grant No. 2022A1515110656). We would like to acknowledge Suzhou Panomix for providing help. We thank SHANGHAI BIOTREE BIOMEDICAL TECHNOLOGY CO., LTD for LC MS/MS and UHPLC-MRM-MS analysis. We also thank Novogene for assistance in RNAseq experiment.
Funding
This work was supported by the National Nature Science Foundation of China (Grant No. 82172751), China Postdoctoral Science Foundation (Grant No. 2021M701625), President Foundation of Nanfang Hospital, Southern Medical University (Grant No. 2021C003), and Guangdong Natural Science Foundation (Grant No. 2022A1515110656).
Author information
Authors and Affiliations
Contributions
QS and MS contributed equally to this work. QS, MS, SZ, LX and LL were responsible for the experimental design and data interpretation. QS, MS and SZ and YL performed the experiments. QS wrote the manuscript. DZ, JS, ZG and LW have verified the underlying data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Q., Shen, M., Zhu, S. et al. Targeting NAD+ metabolism of hepatocellular carcinoma cells by lenvatinib promotes M2 macrophages reverse polarization, suppressing the HCC progression. Hepatol Int 17, 1444–1460 (2023). https://doi.org/10.1007/s12072-023-10544-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10544-7